A non-injected opioid analgesia protocol for acute pain crisis in adolescents and adults with sickle cell disease
- PMID: 35419195
- PMCID: PMC8998522
- DOI: 10.1177/20494637211033814
A non-injected opioid analgesia protocol for acute pain crisis in adolescents and adults with sickle cell disease
Abstract
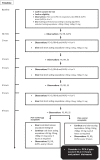
Initial management of the acute pain crisis (APC) of sickle cell disease (SCD) is often unsatisfactory, and might be improved by developing a standardised analgesia protocol. Here, we report the first stages in developing a standard oral protocol for adolescents and adults. Initially, we performed a dose finding study to determine the maximal tolerated dose of sublingual fentanyl (MTD SLF) given on arrival in the acute care facility, when combined with repeated doses of oral oxycodone. We used a dose escalation algorithm with two dosing ranges based on patient's weight (<50 kg or >50 kg). We also made a preliminary evaluation of the safety and efficacy of the protocol. The study took place in a large tertiary centre in London, UK. Ninety patients in the age range 14-60 years were pre-consented and 31 treatment episodes were evaluated. The first 21 episodes constituted the dose escalation study, establishing the MTD SLF at 600 mcg (>50 kg) or 400 mcg (<50 kg). Further evaluation of the protocol indicated no evidence of severe opioid toxicity, nor increased incidence of acute chest syndrome (ACS). Between 0 and 6 hours, the overall gradient of reduction of visual analogue pain score (visual analogue scale (VAS)) was 0.32 centimetres (cm) per hour (95% confidence interval (CI) = 0.20 to 0.44, p < 0.001). For episodes on MTD SLF, there was median (interquartile range (IQR)) reduction in VAS score of 2.8 cm (0-4.2) and 59% had at least a 2.6-cm reduction. These results are supportive of further evaluation of this protocol for acute analgesia of APC in a hospital setting and potentially for supervised home management.
Keywords: Sickle; VOC; analgesia; crisis; opioid; pain.
© The British Pain Society 2021.
Conflict of interest statement
Conflict of interests: PT has received unrestricted grants from Kyowa Kirin and Napp Pharmaceuticals to undertake the study.
Figures


References
-
- Platt OS, Thorington BD, Brambilla DJ, et al.. Pain in sickle cell disease. Rates and risk factors. New Engl J Med 1991; 325: 11–16. - PubMed
-
- Yawn BP, Buchanan GR, Afenyi-Annan AN, et al.. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA 2014; 312: 1033–1048. - PubMed
-
- Gillis VL, Senthinathan A, Dzingina M, et al.. Management of an acute painful sickle cell episode in hospital: summary of NICE guidance. BMJ 2012; 344: e4063. - PubMed
LinkOut - more resources
Full Text Sources
