MR fingerprinting of the prostate
- PMID: 35419668
- PMCID: PMC10288492
- DOI: 10.1007/s10334-022-01012-8
MR fingerprinting of the prostate
Abstract
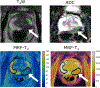
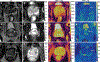
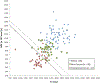
Multiparametric magnetic resonance imaging (mpMRI) has been adopted as the key tool for detection, localization, characterization, and risk stratification of patients suspected to have prostate cancer. Despite advantages over systematic biopsy, the interpretation of prostate mpMRI has limitations including a steep learning curve, leading to considerable interobserver variation. There is growing interest in clinical translation of quantitative imaging techniques for more objective lesion assessment. However, traditional mapping techniques are slow, precluding their use in the clinic. Magnetic resonance fingerprinting (MRF) is an efficient approach for quantitative maps of multiple tissue properties simultaneously. The T1 and T2 values obtained with MRF have been validated with phantom studies as well as in normal volunteers and patients. Studies have shown that MRF-derived T1 and T2 along with ADC values are all significant independent predictors in the differentiation between normal prostate tissue and prostate cancer, and hold promise in differentiating low and intermediate/high-grade cancers. This review seeks to introduce the basics of the prostate MRF technique, discuss the potential applications of prostate MRF for the characterization of prostate cancer, and describes ongoing areas of research.
Keywords: Magnetic resonance fingerprinting; Magnetic resonance imaging; Prostate; Quantitative imaging.
© 2022. The Author(s), under exclusive licence to European Society for Magnetic Resonance in Medicine and Biology (ESMRMB).
Conflict of interest statement
Conflict of Interest: Authors Lo, Panda, Jiang, Ahad, Gulani, and Seiberlich have received research grants from Siemens Healthineers, and authors Jiang, Gulani, and Seiberlich have received royalties from Siemens Healthineers for MRF. Author Lo is currently an employee of Siemens Healthineers.
Figures

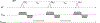


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

