Importance of chemical polymorphism in modern crop protection
- PMID: 35419941
- PMCID: PMC9321084
- DOI: 10.1002/ps.6919
Importance of chemical polymorphism in modern crop protection
Abstract
The development of agrochemical products faces many scientific challenges. After selection of an agrochemical candidate its properties will have to be optimized to guarantee best bioavailability and stability under many different conditions in various formulation types. These challenges are influenced by the solid-state properties of the active ingredient and this makes the selection of an optimized solid-state form of modern agrochemicals at early development stages very valuable. The increasing awareness of the solid state of agrochemicals is reflected in the importance of polymorphism patent applications, which may enhance the risk of litigations. This review aims to present strategies for the solid-form selection process of agrochemical development candidates. It introduces the different techniques for crystallization and analytics and demonstrates the influence of the solid state on different formulation types. © 2022 The Authors. Pest Management Science published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: chemical polymorphism; crystallization; fungicides; herbicides; insecticides; organic solid state; polymorphism screening; solid-state selection.
© 2022 The Authors. Pest Management Science published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Figures






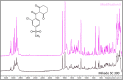
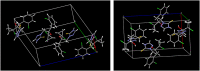

References
-
- Mitscherlich E, On the relationship between crystalline form and composition, I. Note on arsenates and phosphates. Ann Chim Phys 19:350–419 (1822).
-
- Bernstein J, Polymorphism in Molecular Crystals. Clarendon Press, Oxford, UK: (2002).
-
- Hilfiker R, Polymorphism in the Pharmaceutical Industry. Wiley‐VCH, Weinheim: (2006).
-
- Brittain HG, Polymorphism in Pharmaceutical Solids. Informa Healthcare, New York, NY: (2007).
-
- Grunenberg A, Polymorphie und thermische analyse pharmazeutischer Wirkstoffe. Pharm in unserer Zeit 26:224–231 (1997). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

