Psoralen mapping reveals a bacterial genome supercoiling landscape dominated by transcription
- PMID: 35420137
- PMCID: PMC9071471
- DOI: 10.1093/nar/gkac244
Psoralen mapping reveals a bacterial genome supercoiling landscape dominated by transcription
Abstract
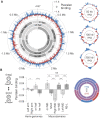
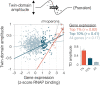
DNA supercoiling is a key regulator of all DNA metabolic processes including replication, transcription, and recombination, yet a reliable genomic assay for supercoiling is lacking. Here, we present a robust and flexible method (Psora-seq) to measure whole-genome supercoiling at high resolution. Using this tool in Escherichia coli, we observe a supercoiling landscape that is well correlated to transcription. Supercoiling twin-domains generated by RNA polymerase complexes span 25 kb in each direction - an order of magnitude farther than previous measurements in any organism. Thus, ribosomal and many other highly expressed genes strongly affect the topology of about 40 neighboring genes each, creating highly integrated gene circuits. Genomic patterns of supercoiling revealed by Psora-seq could be aptly predicted from modeling based on gene expression levels alone, indicating that transcription is the major determinant of chromosome supercoiling. Large-scale supercoiling patterns were highly symmetrical between left and right chromosome arms (replichores), indicating that DNA replication also strongly influences supercoiling. Skew in the axis of symmetry from the natural ori-ter axis supports previous indications that the rightward replication fork is delayed several minutes after initiation. Implications of supercoiling on DNA replication and chromosome domain structure are discussed.
© The Author(s) 2022. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures




References
-
- Masse E., Drolet M.. Escherichia coli DNA topoisomerase i inhibits R-loop formation by relaxing transcription-induced negative supercoiling. J. Biol. Chem. 1999; 274:16659–16664. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

