Hydrogen Atom Transfer Driven Enantioselective Minisci Reaction of Alcohols
- PMID: 35420220
- PMCID: PMC9321721
- DOI: 10.1002/anie.202200266
Hydrogen Atom Transfer Driven Enantioselective Minisci Reaction of Alcohols
Abstract
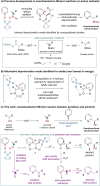
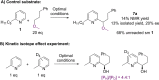
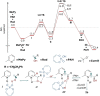
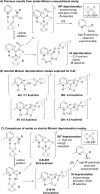
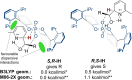
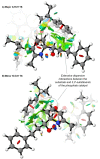
Catalytic enantioselective Minisci reactions have recently been developed but all instances so far utilize α-amino radical coupling partners. We report a substantial evolution of the enantioselective Minisci reaction that enables α-hydroxy radicals to be used, providing valuable enantioenriched secondary alcohol products. This is achieved through the direct oxidative coupling of two C-H bonds on simple alcohol and pyridine partners through a hydrogen atom transfer (HAT)-driven approach: a challenging process to achieve due to the numerous side reactions that can occur. Our approach is highly regioselective as well as highly enantioselective. Dicumyl peroxide, upon irradiation with 390 nm light, serves as both HAT reagent and oxidant whilst selectivity is controlled by use of a chiral phosphoric acid catalyst. Computational and experimental evidence provide mechanistic insight as to the origin of selectivity, revealing a stereodetermining deprotonation step distinct from the analogous reaction of amide-containing substrates.
Keywords: Asymmetric Catalysis; Chiral Phosphoric Acids; Heterocycles; Minisci Reaction; Organocatalysis.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Vitaku E., Smith D. T., Njardarson J. T., J. Med. Chem. 2014, 57, 10257–10274. - PubMed
-
- None
-
- Minisci F., Vismara E., Fontana F., Heterocycles 1989, 28, 489–519;
-
- Minisci F., Fontana F., Vismara E., J. Heterocycl. Chem. 1990, 27, 79–96;
-
- Duncton M. A. J., MedChemComm 2011, 2, 1135–1161;
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

