Catalytic Decomposition of Long-Chain Olefins to Propylene via Isomerization-Metathesis Using Latent Bicyclic (Alkyl)(Amino)Carbene-Ruthenium Olefin Metathesis Catalysts
- PMID: 35420225
- PMCID: PMC9400880
- DOI: 10.1002/anie.202204413
Catalytic Decomposition of Long-Chain Olefins to Propylene via Isomerization-Metathesis Using Latent Bicyclic (Alkyl)(Amino)Carbene-Ruthenium Olefin Metathesis Catalysts
Abstract
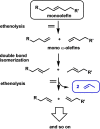
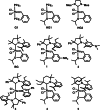
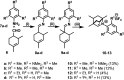
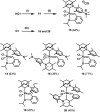
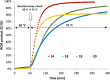
One of the most exciting scientific challenges today is the catalytic degradation of non-biodegradable polymers into value-added chemical feedstocks. The mild pyrolysis of polyolefins, including high-density polyethylene (HDPE), results in pyrolysis oils containing long-chain olefins as major products. In this paper, novel bicyclic (alkyl)(amino)carbene ruthenium (BICAAC-Ru) temperature-activated latent olefin metathesis catalysts, which can be used for catalytic decomposition of long-chain olefins to propylene are reported. These thermally stable catalysts show significantly higher selectivity to propylene at a reaction temperature of 75 °C compared to second generation Hoveyda-Grubbs or CAAC-Ru catalysts under ethenolysis conditions. The conversion of long-chain olefins (e.g., 1-octadecene or methyl oleate) to propylene via isomerization-metathesis is performed by using a (RuHCl)(CO)(PPh3 )3 isomerization co-catalyst. The reactions can be carried out at a BICAAC-Ru catalyst loading as low as 1 ppm at elevated reaction temperature (75 °C). The observed turnover number and turnover frequency are as high as 55 000 and 10 000 molpropylene molcatalyst -1 h-1 , respectively.
Keywords: BICAAC; Hydrocarbon Decomposition; ISOMET; Metathesis; Propylene; Ruthenium.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
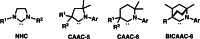


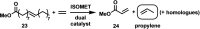
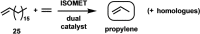
References
-
- “Catalysis Definition in Chemistry,” can be found under https://www.thoughtco.com/definition-of-catalyst-604402%0D%0A, 10.1021/nn8008245. - DOI
-
- V. Haensel, Catalysis: Significance in Industry and Relevance in Chemical Education, 1982.
-
- Sustainable Catalysis: Energy-Efficient Reactions and Applications (Eds.: Luque R., L.-Y. Lam F.), Wiley-VCH, Weinheim, 2018,
- 10.1002/9783527693030. - DOI
-
- Akdogan Z., Guven B., Environ. Pollut. 2019, 254, 113011. - PubMed
-
- Gholami Z., Gholami F., Energies 2021, 14, 8190.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

