Extracellular Vesicles Regulate Biofilm Formation and Yeast-to-Hypha Differentiation in Candida albicans
- PMID: 35420476
- PMCID: PMC9239257
- DOI: 10.1128/mbio.00301-22
Extracellular Vesicles Regulate Biofilm Formation and Yeast-to-Hypha Differentiation in Candida albicans
Abstract
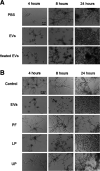
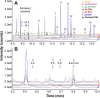
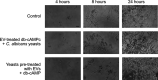
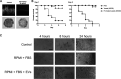
In this study, we investigated the influence of fungal extracellular vesicles (EVs) during biofilm formation and morphogenesis in Candida albicans. Using crystal violet staining and scanning electron microscopy (SEM), we demonstrated that C. albicans EVs inhibited biofilm formation in vitro. By time-lapse microscopy and SEM, we showed that C. albicans EV treatment stopped filamentation and promoted pseudohyphae formation with multiple budding sites. The ability of C. albicans EVs to regulate dimorphism was further compared to EVs isolated from different C. albicans strains, Saccharomyces cerevisiae, and Histoplasma capsulatum. C. albicans EVs from distinct strains inhibited yeast-to-hyphae differentiation with morphological changes occurring in less than 4 h. EVs from S. cerevisiae and H. capsulatum modestly reduced morphogenesis, and the effect was evident after 24 h of incubation. The inhibitory activity of C. albicans EVs on phase transition was promoted by a combination of lipid compounds, which were identified by gas chromatography-tandem mass spectrometry analysis as sesquiterpenes, diterpenes, and fatty acids. Remarkably, C. albicans EVs were also able to reverse filamentation. Finally, C. albicans cells treated with C. albicans EVs for 24 h lost their capacity to penetrate agar and were avirulent when inoculated into Galleria mellonella. Our results indicate that fungal EVs can regulate yeast-to-hypha differentiation, thereby inhibiting biofilm formation and attenuating virulence. IMPORTANCE The ability to undergo morphological changes during adaptation to distinct environments is exploited by Candida albicans and has a direct impact on biofilm formation and virulence. Morphogenesis is controlled by a diversity of stimuli, including osmotic stress, pH, starvation, presence of serum, and microbial components, among others. Apart from external inducers, C. albicans also produces autoregulatory substances. Farnesol and tyrosol are examples of quorum-sensing molecules (QSM) released by C. albicans to regulate yeast-to-hypha conversion. Here, we demonstrate that fungal EVs are messengers impacting biofilm formation, morphogenesis, and virulence in C. albicans. The major players exported in C. albicans EVs included sesquiterpenes, diterpenes, and fatty acids. The understanding of how C. albicans cells communicate to regulate physiology and pathogenesis can lead to novel therapeutic tools to combat candidiasis.
Keywords: Candida albicans; biofilm; extracellular vesicles; lipids; yeast-to-hypha inhibition.
Conflict of interest statement
The authors declare a conflict of interest. Dr. Maurizio Del Poeta, M.D. is a Co-Founder and Chief Scientific Officer (CSO) of MicroRid Technologies Inc.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

