Aging Microbiota-Gut-Brain Axis in Stroke Risk and Outcome
- PMID: 35420913
- PMCID: PMC9674376
- DOI: 10.1161/CIRCRESAHA.122.319983
Aging Microbiota-Gut-Brain Axis in Stroke Risk and Outcome
Abstract
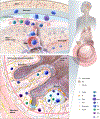
The microbiota-gut-brain-axis (MGBA) is a bidirectional communication network between gut microbes and their host. Many environmental and host-related factors affect the gut microbiota. Dysbiosis is defined as compositional and functional alterations of the gut microbiota that contribute to the pathogenesis, progression and treatment responses to disease. Dysbiosis occurs when perturbations of microbiota composition and function exceed the ability of microbiota and its host to restore a symbiotic state. Dysbiosis leads to dysfunctional signaling of the MGBA, which regulates the development and the function of the host's immune, metabolic, and nervous systems. Dysbiosis-induced dysfunction of the MGBA is seen with aging and stroke, and is linked to the development of common stroke risk factors such as obesity, diabetes, and atherosclerosis. Changes in the gut microbiota are also seen in response to stroke, and may impair recovery after injury. This review will begin with an overview of the tools used to study the MGBA with a discussion on limitations and potential experimental confounders. Relevant MGBA components are introduced and summarized for a better understanding of age-related changes in MGBA signaling and its dysfunction after stroke. We will then focus on the relationship between the MGBA and aging, highlighting that all components of the MGBA undergo age-related alterations that can be influenced by or even driven by the gut microbiota. In the final section, the current clinical and preclinical evidence for the role of MGBA signaling in the development of stroke risk factors such as obesity, diabetes, hypertension, and frailty are summarized, as well as microbiota changes with stroke in experimental and clinical populations. We conclude by describing the current understanding of microbiota-based therapies for stroke including the use of pre-/pro-biotics and supplementations with bacterial metabolites. Ongoing progress in this new frontier of biomedical sciences will lead to an improved understanding of the MGBA's impact on human health and disease.
Keywords: aging; brain-gut axis; dysbiosis; microbiota; risk factors; stroke.
Conflict of interest statement
Conflict of Interest
None.
Figures







References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

