Benchmarking of deep learning algorithms for 3D instance segmentation of confocal image datasets
- PMID: 35421081
- PMCID: PMC9009699
- DOI: 10.1371/journal.pcbi.1009879
Benchmarking of deep learning algorithms for 3D instance segmentation of confocal image datasets
Abstract
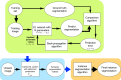
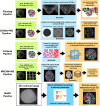
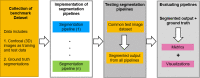
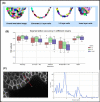
Segmenting three-dimensional (3D) microscopy images is essential for understanding phenomena like morphogenesis, cell division, cellular growth, and genetic expression patterns. Recently, deep learning (DL) pipelines have been developed, which claim to provide high accuracy segmentation of cellular images and are increasingly considered as the state of the art for image segmentation problems. However, it remains difficult to define their relative performances as the concurrent diversity and lack of uniform evaluation strategies makes it difficult to know how their results compare. In this paper, we first made an inventory of the available DL methods for 3D cell segmentation. We next implemented and quantitatively compared a number of representative DL pipelines, alongside a highly efficient non-DL method named MARS. The DL methods were trained on a common dataset of 3D cellular confocal microscopy images. Their segmentation accuracies were also tested in the presence of different image artifacts. A specific method for segmentation quality evaluation was adopted, which isolates segmentation errors due to under- or oversegmentation. This is complemented with a 3D visualization strategy for interactive exploration of segmentation quality. Our analysis shows that the DL pipelines have different levels of accuracy. Two of them, which are end-to-end 3D and were originally designed for cell boundary detection, show high performance and offer clear advantages in terms of adaptability to new data.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


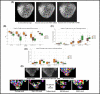
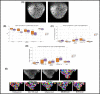

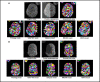


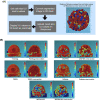









References
-
- Thomas RM, John J. A review on cell detection and segmentation in microscopic images. 2017 International Conference on Circuit, Power and Computing Technologies (ICCPCT). IEEE. 2017:1–5. doi: 10.1109/ICCPCT.2017.8074189 - DOI
-
- Hafiz AM, Bhat GM. A survey on instance segmentation: state of the art. Int J Multimed Inf Retr. 2020;9:171–89. doi: 10.1007/s13735-020-00195-x - DOI
-
- Lei T, Wang R, Wan Y, Du X, Meng H, Nandi A. Medical Image Segmentation Using Deep Learning: A Survey. arXiv. 2020;abs/2009.13120.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

