Pembrolizumab Monotherapy for Previously Untreated Advanced Hepatocellular Carcinoma: Data from the Open-Label, Phase II KEYNOTE-224 Trial
- PMID: 35421228
- PMCID: PMC9784157
- DOI: 10.1158/1078-0432.CCR-21-3807
Pembrolizumab Monotherapy for Previously Untreated Advanced Hepatocellular Carcinoma: Data from the Open-Label, Phase II KEYNOTE-224 Trial
Abstract
Purpose: KEYNOTE-224 cohort 1 demonstrated that pembrolizumab was efficacious and tolerable in patients with advanced hepatocellular carcinoma (HCC) previously treated with sorafenib. We report results from KEYNOTE-224 (NCT02702414) cohort 2, which enrolled patients with advanced HCC and no prior systemic therapy.
Patients and methods: KEYNOTE-224 was an open-label, multicountry phase II trial. Eligible patients in cohort 2 had advanced HCC not amenable or refractory to locoregional therapy and not previously treated with systemic therapy. Patients received pembrolizumab 200 mg intravenously every 3 weeks for ≤2 years. Primary endpoint was objective response rate (ORR) by central imaging review per RECIST v1.1. Secondary endpoints included duration of response (DOR), disease control rate (DCR), time to progression (TTP), progression-free survival (PFS), overall survival (OS), and safety/tolerability.
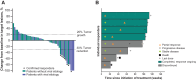
Results: Between September 4, 2018, and February 20, 2019, 51 patients were allocated in cohort 2. The median time from the first dose to data cutoff (January 19, 2021) was 27 months (range, 23-29). ORR was 16% [95% confidence interval (CI), 7-29] and was similar across key subgroups. Median DOR was 16 months (range, 3-24+), and DCR was 57%. The median PFS was 4 months (95% CI, 2-8), and median TTP was 4 months (95% CI, 3-9). Median OS was 17 months (95% CI, 8-23). Grade ≥3 treatment-related adverse events occurred in 16% of patients.
Conclusions: In patients with advanced HCC with no prior systemic therapy, pembrolizumab provided durable antitumor activity, promising OS, and had a safety profile consistent with previous observations. These findings support further evaluation of pembrolizumab-based regimens for HCC.
Trial registration: ClinicalTrials.gov NCT03867084 NCT04246177 NCT02702414.
©2022 The Authors; Published by the American Association for Cancer Research.
Figures


Comment in
- Clin Cancer Res. 28:2475.
- Clin Cancer Res. 28:2475.
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. - PubMed
-
- Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2021;7:6. - PubMed
-
- Global Burden of Disease Liver Cancer Collaboration, Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the Global Burden of Disease Study 2015. JAMA Oncol 2017;3:1683–91. - PMC - PubMed
-
- Cheng A-L, Kang Y-K, Chen Z, Tsao C-J, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomized, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25–34. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

