Ligand-receptor promiscuity enables cellular addressing
- PMID: 35421362
- PMCID: PMC10897978
- DOI: 10.1016/j.cels.2022.03.001
Ligand-receptor promiscuity enables cellular addressing
Abstract
In multicellular organisms, secreted ligands selectively activate, or "address," specific target cell populations to control cell fate decision-making and other processes. Key cell-cell communication pathways use multiple promiscuously interacting ligands and receptors, provoking the question of how addressing specificity can emerge from molecular promiscuity. To investigate this issue, we developed a general mathematical modeling framework based on the bone morphogenetic protein (BMP) pathway architecture. We find that promiscuously interacting ligand-receptor systems allow a small number of ligands, acting in combinations, to address a larger number of individual cell types, defined by their receptor expression profiles. Promiscuous systems outperform seemingly more specific one-to-one signaling architectures in addressing capability. Combinatorial addressing extends to groups of cell types, is robust to receptor expression noise, grows more powerful with increases in the number of receptor variants, and is maximized by specific biochemical parameter relationships. Together, these results identify design principles governing cellular addressing by ligand combinations.
Keywords: BMP; bone morphogenetic protein; cell-type specificity; combinatorial signaling; communication systems; information theory; ligand-receptor interactions; promiscuity; signal processing; signaling pathways.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests C.J.S. is presently at the University of Illinois College of Medicine. H.K. is currently at the Department of Biomedical Engineering at Boston University. The authors have a patent related to this work (U.S. patent number 10,527,631).
Figures
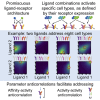
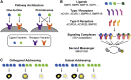



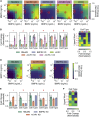


Comment in
-
The context-dependent, combinatorial logic of BMP signaling.Cell Syst. 2022 May 18;13(5):388-407.e10. doi: 10.1016/j.cels.2022.03.002. Epub 2022 Apr 13. Cell Syst. 2022. PMID: 35421361 Free PMC article.
References
-
- Albilia J.B., Tenenbaum H.C., Clokie C.M.L., Walt D.R., Baker G.I., Psutka D.J., Backstein D., Peel S.A.F. Serum levels of BMP-2, 4, 7 and AHSG in patients with degenerative joint disease requiring total arthroplasty of the hip and temporomandibular joints. J. Orthop. Res. 2013;31:44–52. - PubMed
-
- Baur S.T., Mai J.J., Dymecki S.M. Combinatorial signaling through BMP receptor IB and GDF5: shaping of the distal mouse limb and the genetics of distal limb diversity. Development. 2000;127:605–619. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

