Mechanotransduction-induced glycolysis epigenetically regulates a CXCL1-dominant angiocrine signaling program in liver sinusoidal endothelial cells in vitro and in vivo
- PMID: 35421427
- PMCID: PMC9391258
- DOI: 10.1016/j.jhep.2022.03.029
Mechanotransduction-induced glycolysis epigenetically regulates a CXCL1-dominant angiocrine signaling program in liver sinusoidal endothelial cells in vitro and in vivo
Abstract
Background & aims: Liver sinusoidal endothelial cells (LSECs) are ideally situated to sense stiffness and generate angiocrine programs that potentially regulate liver fibrosis and portal hypertension. We explored how specific focal adhesion (FA) proteins parlay LSEC mechanotransduction into stiffness-induced angiocrine signaling in vitro and in vivo.
Methods: Primary human and murine LSECs were placed on gels with incremental stiffness (0.2 kPa vs. 32 kPa). Cell response was studied by FA isolation, actin polymerization assay, RNA-sequencing and electron microscopy. Glycolysis was assessed using radioactive tracers. Epigenetic regulation of stiffness-induced genes was analyzed by chromatin-immunoprecipitation (ChIP) analysis of histone activation marks, ChIP sequencing and circularized chromosome conformation capture (4C). Mice with LSEC-selective deletion of glycolytic enzymes (Hk2fl/fl/Cdh5cre-ERT2) or treatment with the glycolysis inhibitor 3PO were studied in portal hypertension (partial ligation of the inferior vena cava, pIVCL) and early liver fibrosis (CCl4) models.
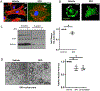
Results: Glycolytic enzymes, particularly phosphofructokinase 1 isoform P (PFKP), are enriched in isolated FAs from LSECs on gels with incremental stiffness. Stiffness resulted in PFKP recruitment to FAs, which paralleled an increase in glycolysis. Glycolysis was associated with expansion of actin dynamics and was attenuated by inhibition of integrin β1. Inhibition of glycolysis attenuated a stiffness-induced CXCL1-dominant angiocrine program. Mechanistically, glycolysis promoted CXCL1 expression through nuclear pore changes and increases in NF-kB translocation. Biochemically, this CXCL1 expression was mediated through spatial re-organization of nuclear chromatin resulting in formation of super-enhancers, histone acetylation and NF-kB interaction with the CXCL1 promoter. Hk2fl/fl/Cdh5cre-ERT2 mice showed attenuated neutrophil infiltration and portal hypertension after pIVCL. 3PO treatment attenuated liver fibrosis in a CCl4 model.
Conclusion: Glycolytic enzymes are involved in stiffness-induced angiocrine signaling in LSECs and represent druggable targets in early liver disease.
Lay summary: Treatment options for liver fibrosis and portal hypertension still represent an unmet need. Herein, we uncovered a novel role for glycolytic enzymes in promoting stiffness-induced angiocrine signaling, which resulted in inflammation, fibrosis and portal hypertension. This work has revealed new targets that could be used in the prevention and treatment of liver fibrosis and portal hypertension.
Keywords: CXCL1; actin polymerization; angiocrine signaling; glycolysis; liver sinusoidal endothelial cells; mechanosensing; nuclear pores; portal hypertension.
Copyright © 2022 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflicts of interest The authors declare no conflicts of interest that pertain to this work. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures







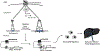
References
-
- Greuter T, Shah VH. Hepatic sinusoids in liver injury, inflammation, and fibrosis: new pathophysiological insights. J Gastroenterol. 2016;51(6):511–9. - PubMed
-
- Dauphinee SM, Karsan A. Lipopolysaccharide signaling in endothelial cells. Lab Invest. 2006;86(1):9–22. - PubMed
-
- Schrage A, Wechsung K, Neumann K, Schumann M, Schulzke JD, Engelhardt B, et al. Enhanced T cell transmigration across the murine liver sinusoidal endothelium is mediated by transcytosis and surface presentation of chemokines. Hepatology. 2008;48(4):1262–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

