Immunogenicity of the mRNA-1273 COVID-19 vaccine in adult patients with inborn errors of immunity
- PMID: 35421449
- PMCID: PMC8996444
- DOI: 10.1016/j.jaci.2022.04.002
Immunogenicity of the mRNA-1273 COVID-19 vaccine in adult patients with inborn errors of immunity
Abstract
Background: Patients with inborn errors of immunity (IEI) are at increased risk of severe coronavirus disease-2019 (COVID-19). Effective vaccination against COVID-19 is therefore of great importance in this group, but little is known about the immunogenicity of COVID-19 vaccines in these patients.
Objectives: We sought to study humoral and cellular immune responses after mRNA-1273 COVID-19 vaccination in adult patients with IEI.
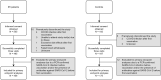
Methods: In a prospective, controlled, multicenter study, 505 patients with IEI (common variable immunodeficiency [CVID], isolated or undefined antibody deficiencies, X-linked agammaglobulinemia, combined B- and T-cell immunodeficiency, phagocyte defects) and 192 controls were included. All participants received 2 doses of the mRNA-1273 COVID-19 vaccine. Levels of severe acute respiratory syndrome coronavirus-2-specific binding antibodies, neutralizing antibodies, and T-cell responses were assessed at baseline, 28 days after first vaccination, and 28 days after second vaccination.
Results: Seroconversion rates in patients with clinically mild antibody deficiencies and phagocyte defects were similar to those in healthy controls, but seroconversion rates in patients with more severe IEI, such as CVID and combined B- and T-cell immunodeficiency, were lower. Binding antibody titers correlated well to the presence of neutralizing antibodies. T-cell responses were comparable to those in controls in all IEI cohorts, with the exception of patients with CVID. The presence of noninfectious complications and the use of immunosuppressive drugs in patients with CVID were negatively correlated with the antibody response.
Conclusions: COVID-19 vaccination with mRNA-1273 was immunogenic in mild antibody deficiencies and phagocyte defects and in most patients with combined B- and T-cell immunodeficiency and CVID. Lowest response was detected in patients with X-linked agammaglobulinemia and in patients with CVID with noninfectious complications. The assessment of longevity of immune responses in these vulnerable patient groups will guide decision making for additional vaccinations.
Keywords: CID; CVID; Inborn errors of immunity; SARS-CoV-2; T-cell response; XLA; antibody response; immunogenicity; mRNA-1273 COVID-19 vaccine; primary immunodeficiency disorders.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

