Physiologically-based pharmacokinetic model-based translation of OATP1B-mediated drug-drug interactions from coproporphyrin I to probe drugs
- PMID: 35421902
- PMCID: PMC9199885
- DOI: 10.1111/cts.13272
Physiologically-based pharmacokinetic model-based translation of OATP1B-mediated drug-drug interactions from coproporphyrin I to probe drugs
Abstract
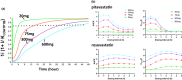
The accurate prediction of OATP1B-mediated drug-drug interactions (DDIs) is challenging for drug development. Here, we report a physiologically-based pharmacokinetic (PBPK) model analysis for clinical DDI data generated in heathy subjects who received oral doses of cyclosporin A (CysA; 20 and 75 mg) as an OATP1B inhibitor, and the probe drugs (pitavastatin, rosuvastatin, and valsartan). PBPK models of CysA and probe compounds were combined assuming inhibition of hepatic uptake of endogenous coproporphyrin I (CP-I) by CysA. In vivo Ki of unbound CysA for OATP1B (Ki,OATP1B ), and the overall intrinsic hepatic clearance per body weight of CP-I (CLint,all,unit ) were optimized to account for the CP-I data (Ki,OATP1B , 0.536 ± 0.041 nM; CLint,all,unit , 41.9 ± 4.3 L/h/kg). DDI simulation using Ki,OATP1B reproduced the dose-dependent effect of CysA (20 and 75 mg) and the dosing interval (1 and 3 h) on the time profiles of blood concentrations of pitavastatin and rosuvastatin, but DDI simulation using in vitro Ki,OATP1B failed. The Cluster Gauss-Newton method was used to conduct parameter optimization using 1000 initial parameter sets for the seven pharmacokinetic parameters of CP-I (β, CLint, all , Fa Fg , Rdif , fbile , fsyn , and vsyn ), and Ki,OATP1B and Ki,MRP2 of CysA. Based on the accepted 546 parameter sets, the range of CLint, all and Ki,OATP1B was narrowed, with coefficients of variation of 12.4% and 11.5%, respectively, indicating that these parameters were practically identifiable. These results suggest that PBPK model analysis of CP-I is a promising translational approach to predict OATP1B-mediated DDIs in drug development.
© 2022 The Authors. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
Tadayuki Takashima is an employee of Asahi Kasei Pharma; Kenta Yoshida and Jialin Mao are employees of Genentech; Yurong Lai is an employee of Gilead Sciences; Kunal Taskar and Maciej J. Zamek‐Gliszczynski are employees of GlaxoSmithKline; Kevin Rockich is an employee of Incyte Research Institute; Xiaoyan Chu is an employee of Merck & Co., Inc.; Yoshiyuki Yamaura is an employee of Ono Pharmaceuticals; and Hideki Hirabayashi is an employee of Takeda. All other authors declared no competing interests for this work.
Figures



References
-
- Watanabe T, Kusuhara H, Sugiyama Y. Application of physiologically based pharmacokinetic modeling and clearance concept to drugs showing transporter‐mediated distribution and clearance in humans. J Pharmacokinet Pharmacodyn. 2010;37:575‐590. - PubMed
-
- Watanabe T, Kusuhara H, Maeda K, Shitara Y, Sugiyama Y. Physiologically based pharmacokinetic modeling to predict transporter‐mediated clearance and distribution of pravastatin in humans. J Pharmacol Exp Ther. 2009;328:652‐662. - PubMed
-
- Yoshikado T, Yoshida K, Kotani N, et al. Quantitative analyses of hepatic OATP‐mediated interactions between statins and inhibitors using PBPK modeling with a parameter optimization method. Clin Pharmacol Ther. 2016;100:513‐523. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

