INFLATE: a protocol for a randomised controlled trial comparing nasal balloon autoinflation to no nasal balloon autoinflation for otitis media with effusion in Aboriginal and Torres Strait Islander children
- PMID: 35421984
- PMCID: PMC9009496
- DOI: 10.1186/s13063-022-06145-8
INFLATE: a protocol for a randomised controlled trial comparing nasal balloon autoinflation to no nasal balloon autoinflation for otitis media with effusion in Aboriginal and Torres Strait Islander children
Abstract
Background: Otitis media with effusion (OME) is common and occurs at disproportionately higher rates among Indigenous children. Left untreated, OME can negatively affect language, development, learning, and health and wellbeing throughout the life-course. Currently, OME care includes observation for 3 months followed by consideration of surgical ventilation tube insertion. The use of a non-invasive, low-cost nasal balloon autoinflation device has been found beneficial in other populations but has not been investigated among Aboriginal and Torres Strait Islander children.
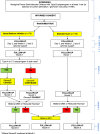
Methods/design: This multi-centre, open-label, randomised controlled trial will determine the effectiveness of nasal balloon autoinflation compared to no nasal balloon autoinflation, for the treatment of OME among Aboriginal and Torres Strait Islander children in Australia. Children aged 3-16 years with unilateral or bilateral OME are being recruited from Aboriginal Health Services and the community. The primary outcome is the proportion of children showing tympanometric improvement of OME at 1 month. Improvement is defined as a change from bilateral type B tympanograms to at least one type A or C1 tympanogram, or from unilateral type B tympanogram to type A or C1 tympanogram in the index ear, without deterioration (type A or C1 to type C2, C3, or B tympanogram) in the contralateral ear. A sample size of 340 children (170 in each group) at 1 month will detect an absolute difference of 15% between groups with 80% power at 5% significance. Anticipating a 15% loss to follow-up, 400 children will be randomised. The primary analysis will be by intention to treat. Secondary outcomes include tympanometric changes at 3 and 6 months, hearing at 3 months, ear health-related quality of life (OMQ-14), and cost-effectiveness. A process evaluation including perspectives of parents or carers, health care providers, and researchers on trial implementation will also be undertaken.
Discussion: INFLATE will answer the important clinical question of whether nasal balloon autoinflation is an effective and acceptable treatment for Aboriginal and Torres Strait Islander children with OME. INFLATE will help fill the evidence gap for safe, low-cost, accessible OME therapies.
Trial registration: Australia New Zealand Clinical Trials Registry ACTRN12617001652369 . Registered on 22 December 2017. The Australia New Zealand Clinical Trials Registry is a primary registry of the WHO ICTRP network and includes all items from the WHO Trial Registration data set. Retrospective registration.
Keywords: Aboriginal and Torres Strait Islander; Children; Ear disease; Indigenous; Nasal autoinflation; Otitis media; Otitis media with effusion; Otolaryngology; Randomised controlled trial.
© 2022. The Author(s).
Conflict of interest statement
JR, DA, GS, CW, CT, LC, and SH work at participating trial sites, in dual roles as researchers and clinicians or managers.
References
-
- Menzies School of Health Research. Otitis media guidelines. In: Otitis Media Technical Advisory Group, editor. 2020.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous