PINK1-mediated Drp1S616 phosphorylation modulates synaptic development and plasticity via promoting mitochondrial fission
- PMID: 35422062
- PMCID: PMC9010405
- DOI: 10.1038/s41392-022-00933-z
PINK1-mediated Drp1S616 phosphorylation modulates synaptic development and plasticity via promoting mitochondrial fission
Abstract
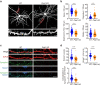
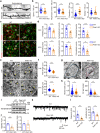
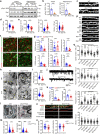
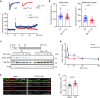
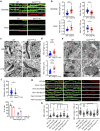
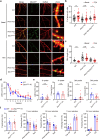
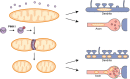
Dynamic change of mitochondrial morphology and distribution along neuronal branches are essential for neural circuitry formation and synaptic efficacy. However, the underlying mechanism remains elusive. We show here that Pink1 knockout (KO) mice display defective dendritic spine maturation, reduced axonal synaptic vesicles, abnormal synaptic connection, and attenuated long-term synaptic potentiation (LTP). Drp1 activation via S616 phosphorylation rescues deficits of spine maturation in Pink1 KO neurons. Notably, mice harboring a knockin (KI) phosphor-null Drp1S616A recapitulate spine immaturity and synaptic abnormality identified in Pink1 KO mice. Chemical LTP (cLTP) induces Drp1S616 phosphorylation in a PINK1-dependent manner. Moreover, phosphor-mimetic Drp1S616D restores reduced dendritic spine localization of mitochondria in Pink1 KO neurons. Together, this study provides the first in vivo evidence of functional regulation of Drp1 by phosphorylation and suggests that PINK1-Drp1S616 phosphorylation coupling is essential for convergence between mitochondrial dynamics and neural circuitry formation and refinement.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

