Microvascular stabilization via blood-brain barrier regulation prevents seizure activity
- PMID: 35422069
- PMCID: PMC9010415
- DOI: 10.1038/s41467-022-29657-y
Microvascular stabilization via blood-brain barrier regulation prevents seizure activity
Abstract
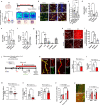
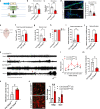
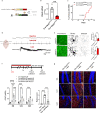
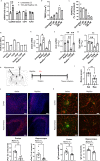
Blood-brain barrier (BBB) dysfunction is associated with worse epilepsy outcomes however the underlying molecular mechanisms of BBB dysfunction remain to be elucidated. Tight junction proteins are important regulators of BBB integrity and in particular, the tight junction protein claudin-5 is the most enriched in brain endothelial cells and regulates size-selectivity at the BBB. Additionally, disruption of claudin-5 expression has been implicated in numerous disorders including schizophrenia, depression and traumatic brain injury, yet its role in epilepsy has not been fully deciphered. Here we report that claudin-5 protein levels are significantly diminished in surgically resected brain tissue from patients with treatment-resistant epilepsy. Concomitantly, dynamic contrast-enhanced MRI in these patients showed widespread BBB disruption. We show that targeted disruption of claudin-5 in the hippocampus or genetic heterozygosity of claudin-5 in mice exacerbates kainic acid-induced seizures and BBB disruption. Additionally, inducible knockdown of claudin-5 in mice leads to spontaneous recurrent seizures, severe neuroinflammation, and mortality. Finally, we identify that RepSox, a regulator of claudin-5 expression, can prevent seizure activity in experimental epilepsy. Altogether, we propose that BBB stabilizing drugs could represent a new generation of agents to prevent seizure activity in epilepsy patients.
© 2022. The Author(s).
Conflict of interest statement
Trinity College Dublin owns an intellectual property portfolio related to the regulation of claudin-5 to treat epilepsy. M.C., C.G., N.H. and C.D. are named inventors. All other authors declare no competing interest.
Figures

Comment in
-
Overcoming Barriers to Improve Treatments in Epilepsy.Epilepsy Curr. 2022 Oct 8;22(5):321-323. doi: 10.1177/15357597221127164. eCollection 2022 Sep-Oct. Epilepsy Curr. 2022. PMID: 36285197 Free PMC article. No abstract available.
References
-
- Epilepsy, http://www.who.int/mediacentre/factsheets/fs999/en/ (2017).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

