Predictors of impaired SARS-CoV-2 immunity in healthcare workers after vaccination with BNT162b2
- PMID: 35422075
- PMCID: PMC9009978
- DOI: 10.1038/s41598-022-10307-8
Predictors of impaired SARS-CoV-2 immunity in healthcare workers after vaccination with BNT162b2
Abstract
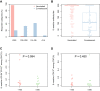
Healthcare workers are at substantially increased risk for infection with SARS-CoV-2. Successful vaccination constitutes a crucial prerequisite to protect this group during the pandemic. Since post vaccination antibody monitoring is not standard of care in all healthcare institutions, data on risk factors of impaired vaccine induced immune response are urgently required. Moreover, there are no data on cellular immune responses in humoral low responders so far. Anti-SARS-CoV-2 spike IgG was assessed after vaccination with BNT162b2 in 1386 employees of three hospitals of a German healthcare provider. Concentrations were compared to those of 45 convalescent employees. Vaccine-induced cellular immunity was measured in employees with reduced humoral response by assessment of frequencies of SARS-CoV-2-reactive CD4+ and CD8+ T cell. Anti-SARS-CoV-2 spike IgG were detected in 99.9% of 1386 healthcare workers after completed vaccination. The median antibody concentration was significantly higher after vaccination than after infection with SARS-CoV-2 (p = 0.0001). 10 subjects (0.7%) generated an IgG concentration < 100 IU/ml, and only two persons (0.1%, solid organ recipients) did not produce detectable antibodies at all. T cell responses of those subjects with submaximal or lacking humoral response were comparable to employees with maximal antibody titers. 50% of those individuals with impaired or lacking humoral immune response were on immunosuppression. Vaccination to SARS-CoV-2 with BNT162b2 is very effective in healthcare workers yielding a seroconversion rate of 99.9%. Immunosuppression is the most important risk factor of an impaired immune response. There was no case of vaccination failure without immunosuppression. Thus, post vaccination antibody monitoring is highly recommendable in those employees with immunosuppression.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

