Results from 22 years of Followup in the Göteborg Randomized Population-Based Prostate Cancer Screening Trial
- PMID: 35422134
- PMCID: PMC9275849
- DOI: 10.1097/JU.0000000000002696
Results from 22 years of Followup in the Göteborg Randomized Population-Based Prostate Cancer Screening Trial
Abstract
Purpose: Our goal was to analyze results from 22 years of followup in the Göteborg randomized prostate cancer (PC) screening trial.
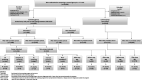
Materials and methods: In December 1994, 20,000 men born 1930-1944 were randomly extracted from the Swedish population register and were randomized (1:1) into either a screening group (SG) or to a control group (CG). Men in the SG were repeatedly invited for biennial prostate specific antigen testing up to an average age of 69 years. Main endpoints were PC incidence and mortality (intention-to-screen principle).
Results: After 22 years, 1,528 men in the SG and 1,124 men in the CG had been diagnosed with PC. In total, 112 PC deaths occurred in the SG and 158 in the CG. Compared with the CG, the SG showed a PC incidence rate ratio (RR) of 1.42 (95% CI, 1.31-1.53) and a PC mortality RR of 0.71 (95% CI, 0.55-0.91). The 22-year cumulative PC mortality rate was 1.55% (95% CI, 1.29-1.86) in the SG and 2.13% (95% CI, 1.83-2.49) in the CG. Correction for nonattendance (Cuzick method) yielded a RR of PC mortality of 0.59 (95% CI, 0.43-0.80). Number needed to invite and number needed to diagnose was estimated to 221 and 9, respectively. PC death risk was increased in the following groups: nontesting men, men entering the program after age 60 and men with >10 years of followup after screening termination.
Conclusions: Prostate specific antigen-based screening substantially decreases PC mortality. However, not attending, starting after age 60 and stopping at age 70 seem to be major pitfalls regarding PC death risk.
Keywords: epidemiology; mass screening; mortality; prostate-specific antigen; prostatic neoplasms.
Conflict of interest statement
Conflict of Interest: Hans Lilja holds patents for intact PSA assays, and is named on a patent application for a statistical method to detect prostate cancer. These patents have been licensed and commercialized as the 4Kscore® by OPKO Health. Dr. Lilja receives royalties from sales of this test and owns stock in OPKO.
Figures
Comment in
-
PSA-Screening in Schweden: Deutlich geringere Prostatakarzinom-Mortalität.Aktuelle Urol. 2023 Feb;54(1):10. doi: 10.1055/a-1925-4156. Epub 2023 Feb 14. Aktuelle Urol. 2023. PMID: 36787767 German. No abstract available.
References
-
- Schroder FH, Hugosson J, Roobol MJ, et al. : Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009; 360: 1320. - PubMed
-
- Kilpelainen TP, Tammela TL, Malila N, et al. : Prostate cancer mortality in the Finnish randomized screening trial. J Natl Cancer Inst 2013; 105: 719. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical