Novel Framework for Treatment Response Evaluation Using PSMA PET/CT in Patients with Metastatic Castration-Resistant Prostate Cancer (RECIP 1.0): An International Multicenter Study
- PMID: 35422442
- PMCID: PMC9635677
- DOI: 10.2967/jnumed.121.263072
Novel Framework for Treatment Response Evaluation Using PSMA PET/CT in Patients with Metastatic Castration-Resistant Prostate Cancer (RECIP 1.0): An International Multicenter Study
Erratum in
-
Erratum.J Nucl Med. 2023 Sep;64(9):1503. J Nucl Med. 2023. PMID: 37657930 Free PMC article. No abstract available.
Abstract
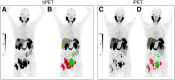
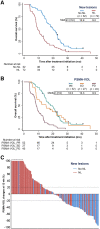
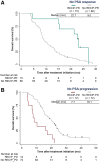
Our objective was to develop version 1.0 of a novel framework for response evaluation criteria in prostate-specific membrane antigen (PSMA) PET/CT (RECIP) and a composite response classification that combines responses by prostate-specific antigen (PSA) measurements and by RECIP 1.0 (PSA + RECIP). Methods: This was an international multicenter, retrospective study. One hundred twenty-four men with metastatic castration-specific prostate cancer (mCRPC) who underwent 177Lu-PSMA therapy and received PSMA PET/CT at baseline and at an interim time point of 12 wk were included. Pairs of baseline interim PET/CT scans were interpreted by consensus among 3 masked readers for appearance of new lesions. Tumor lesions were segmented, and total PSMA-positive tumor volume (PSMA-VOL) was obtained. Appearance of new lesions and changes in PSMA-VOL were combined to develop RECIP 1.0, which included classifications of complete response (RECIP-CR: absence of any PSMA-ligand uptake on interim PET/CT), partial response (RECIP-PR: decline ≥ 30% in PSMA-VOL and no appearance of new lesions), progressive disease (RECIP-PD: increase ≥ 20% in PSMA-VOL and appearance of new lesions), and stable disease (RECIP-SD: any condition but RECIP-PR or RECIP-PD). Changes in PSA levels at 12 wk by Prostate Cancer Working Group Criteria 3 were recorded. PSA + RECIP results were defined as response (PSA decline ≥ 50% or RECIP-PR/CR) or progression (PSA increase ≥ 25% or RECIP-PD). The study's primary outcome measure was the prognostic value of RECIP 1.0 for overall survival (OS). The secondary outcome measure was the prognostic accuracy (C-index) of PSA + RECIP versus PSA responses. Results: Patients with RECIP-PD (n = 39; 8.3 mo) had a shorter OS than patients with stable disease (RECIP-SD) (n = 47; 13.1 mo; P < 0.001) or RECIP-PR (n = 38; 21.7 mo; P < 0.001). In identifying responders and progressors, PSA + RECIP had C-indices superior to those of PSA only: 0.65 versus 0.62 (P = 0.028) and 0.66 versus 0.63 (P = 0.044), respectively. Conclusion: PSMA PET/CT by RECIP 1.0 is prognostic for OS and can be used as a response biomarker to monitor early efficacy of 177Lu-PSMA in men with mCRPC. PSA + RECIP may be used as a novel composite endpoint in mCRPC clinical trial design.
Keywords: 177Lu-PSMA; PSMA PET; interim PET; metastatic castration-resistant prostate cancer; radionuclide treatment.
© 2022 by the Society of Nuclear Medicine and Molecular Imaging.
Figures


References
-
- Hofman MS, Lawrentschuk N, Francis RJ, et al. . Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. 2020;395:1208–1216. - PubMed
-
- FDA approves first PSMA-targeted PET imaging drug for men with prostate cancer. U.S. Food and Drug Administration website. https://www.fda.gov/news-events/press-announcements/fda-approves-first-p.... Published December 1, 2020. Accessed July 21, 2021.
-
- Seitz AK, Rauscher I, Haller B, et al. . Preliminary results on response assessment using 68Ga-HBED-CC-PSMA PET/CT in patients with metastatic prostate cancer undergoing docetaxel chemotherapy. Eur J Nucl Med Mol Imaging. 2018;45:602–612. - PubMed
-
- Clark MS, Packard AT, Johnson DR, Johnson GB. Pitfalls of a mixed metabolic response at PET/CT. Radiographics. 2019;39:1461–1475. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
