18F-PI-2620 Tau PET Improves the Imaging Diagnosis of Progressive Supranuclear Palsy
- PMID: 35422444
- PMCID: PMC9635682
- DOI: 10.2967/jnumed.121.262854
18F-PI-2620 Tau PET Improves the Imaging Diagnosis of Progressive Supranuclear Palsy
Abstract
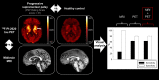
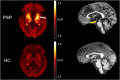
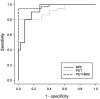
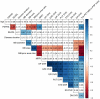
Progressive supranuclear palsy (PSP) is a 4-repeat tauopathy movement disorder that can be imaged by the 18F-labeled tau PET tracer 2-(2-([18F]fluoro)pyridin-4-yl)-9H-pyrrolo[2,3-b:4,5-c']dipyridine (18F-PI-2620). The in vivo diagnosis is currently established on clinical grounds and supported by midbrain atrophy estimation in structural MRI. Here, we investigate whether 18F-PI-2620 tau PET has the potential to improve the imaging diagnosis of PSP. Methods: In this multicenter observational study, dynamic (0-60 min after injection) 18F-PI-2620 PET and structural MRI data for 36 patients with PSP, 22 with PSP-Richardson syndrome, and 14 with a clinical phenotype other than Richardson syndrome (i.e., variant PSP) were analyzed along with data for 10 age-matched healthy controls (HCs). The PET data underwent kinetic modeling, which resulted in distribution volume ratio (DVR) images. These and the MR images were visually assessed by 3 masked experts for typical PSP signs. Furthermore, established midbrain atrophy parameters were measured in structural MR images, and regional DVRs were measured in typical tau-in-PSP target regions in the PET data. Results: Visual assessments discriminated PSP patients and HCs with an accuracy of 63% for MRI and 80% for the combination of MRI and 18F-PI-2620 PET. As compared with patients of the PSP-Richardson syndrome subgroup, those of the variant PSP subgroup profited more in terms of sensitivity from the addition of the visual 18F-PI-2620 PET to the visual MRI information (35% vs. 22%). In quantitative image evaluation, midbrain-to-pons area ratio and globus pallidus DVRs discriminated best between the PSP patients and HCs, with sensitivities and specificities of 83% and 90%, respectively, for MRI and 94% and 100%, respectively, for the combination of MRI and 18F-PI-2620 PET. The gain of sensitivity by adding 18F-PI-2620 PET to MRI data was more marked in clinically less affected patients than in more affected patients (37% vs. 19% for visual, and 16% vs. 12% for quantitative image evaluation). Conclusion: These results provide evidence for an improved imaging-based PSP diagnosis by adding 18F-PI-2620 tau PET to structural MRI. This approach seems to be particularly promising at earlier disease stages and could be of value both for improving early clinical PSP diagnosis and for enriching PSP cohorts for trials of disease-modifying drugs.
Keywords: 18F-PI-2620; 4R-tauopathy; midbrain atrophy; progressive supranuclear palsy; tau PET.
© 2022 by the Society of Nuclear Medicine and Molecular Imaging.
Figures

References
-
- Steele JC, Richardson JC, Olszewski J. Progressive supranuclear palsy: a heterogeneous degeneration involving the brain stem, basal ganglia and cerebellum with vertical gaze and pseudobulbar palsy, nuchal dystonia and dementia. Arch Neurol. 1964;10:333–359. - PubMed
-
- Dickson DW, Ahmed Z, Algom AA, Tsuboi Y, Josephs KA. Neuropathology of variants of progressive supranuclear palsy. Curr Opin Neurol. 2010;23:394–400. - PubMed
-
- Rösler TW, Tayaranian Marvian A, Brendel M, et al. . Four-repeat tauopathies. Prog Neurobiol. 2019;180:101644. - PubMed
-
- Respondek G, Stamelou M, Kurz C, et al. .; Movement Disorder Society-endorsed PSP Study Group. The phenotypic spectrum of progressive supranuclear palsy: a retrospective multicenter study of 100 definite cases. Mov Disord. 2014;29:1758–1766. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
