Blood-based tumor mutational burden as a biomarker for atezolizumab in non-small cell lung cancer: the phase 2 B-F1RST trial
- PMID: 35422531
- PMCID: PMC9117143
- DOI: 10.1038/s41591-022-01754-x
Blood-based tumor mutational burden as a biomarker for atezolizumab in non-small cell lung cancer: the phase 2 B-F1RST trial
Abstract
Tumor mutational burden (TMB) in circulating tumor DNA (ctDNA) has shown promise in predicting benefit from PD-L1/PD-1 inhibitors in retrospective studies. Aiming to assess blood TMB (bTMB) prospectively, we conducted B-F1RST ( NCT02848651 ), an open-label, phase 2 trial that evaluated bTMB as a predictive biomarker for first-line atezolizumab monotherapy in locally advanced or metastatic stage IIIB-IVB non-small cell lung cancer (n = 152). The co-primary endpoints were investigator-assessed objective response rate (ORR) per RECIST version 1.1 and investigator-assessed progression-free survival (PFS) between high and low bTMB subgroups at the pre-defined bTMB ≥ 16 (14.5 mutations per megabase) cutoff. Secondary endpoints included investigator-assessed PFS, overall survival (OS) and duration of response at various bTMB cutoffs, as well as safety. Investigator-assessed PFS in the bTMB ≥ 16 versus bTMB < 16 groups was not statistically significant. However, bTMB ≥ 16 was associated with higher ORR, and ORR improved as bTMB cutoffs increased. No new safety signals were seen. In exploratory analyses, patients with maximum somatic allele frequency (MSAF) < 1% had higher ORR than patients with MSAF ≥ 1%. However, further analysis showed that this effect was driven by better baseline prognostics rather than by MSAF itself. At 36.5-month follow-up, an exploratory analysis of OS found that bTMB ≥ 16 was associated with longer OS than bTMB < 16. Further study and assay optimization will be required to develop bTMB as a predictive, standalone biomarker of immunotherapy or for use in conjunction with other biomarkers.
© 2022. The Author(s).
Conflict of interest statement
The authors declare the following competing interests: E.S.K. has received consulting fees from AstraZeneca, Merck and Roche/Genentech. V.V. has received fees for consulting or serving on advisory boards for Bristol Myers Squibb, Merck, GlaxoSmithKline, Foundation Medicine, AstraZeneca, EMD Serono, Novartis and Novocure. T.M declares no competing interests. C.Y. is an employee of Genentech and holds stock in Roche. S.M.S. is an employee of Genentech and holds stock in Roche. S.H. is employed by Genentech and holds stock in Roche. Y.K.C. has received research grants from AbbVie, Bristol Myers Squibb, Biodesix, Lexent Bio and Freenome and fees for serving on advisory boards for Roche/Genentech, Bristol Myers Squibb, AstraZeneca, Merck, Foundation Medicine, Counsyl, Neogenomics, Guardant Health, Boehringher Ingelheim, Biodesix, ImmuneOncia, Lilly Oncology, Merck, Takeda, Pfizer, Tempus, Lunit and Jazz Pharmaceuticals. T.A.L. has received fees for serving on advisory boards for Jazz Pharmaceuticals, AstraZeneca, EMD Serono, Merck, Blueprint, Debio and Bayer and consultancy for Boehringer Ingelheim, Daiichi Sankyo, Genentech and Jazz Pharmaceuticals. T.A.L. has also served as a non-compensated member of the steering committee for the SAPPHIRE trial, sponsored by Mirati Therapeutics. J.E.D. has received fess for serving on advisory boards for AstraZeneca, Genentech and Janssen. M.L.T., S.R.D. and P.S. declare no competing interests. Y.J. is an employee of and holds stock in Roche. D.S.S. is an employee of Genentech and holds stock in Roche. E.S. is an employee of Genentech and holds stock in Roche. D.A.F. is an employee of Foundation Medicine and holds stock in Roche. S.P. is an employee of Genentech and holds stock in Roche. M.A.S. has received research grants from Genentech, Spectrum, AstraZeneca, Novartis and Daichii Sankyo and has received fees for serving on speaker bureaus for Genentech, AstraZeneca, Bayer, Novartis, Guardant and Amgen.
Figures
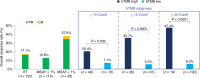
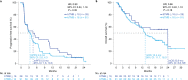


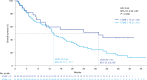
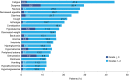




Comment in
-
Prospective insights on the use of bTMB.Nat Rev Clin Oncol. 2022 Jun;19(6):360. doi: 10.1038/s41571-022-00640-2. Nat Rev Clin Oncol. 2022. PMID: 35477773 No abstract available.
References
-
- Kowanetz M, et al. Abstract OA20.01: Tumor mutation burden (TMB) is associated with improved efficacy of atezolizumab in 1L and 2L+ NSCLC patients. J. Thorac. Oncol. 2017;12:S321–S322. doi: 10.1016/j.jtho.2016.11.343. - DOI
-
- Herbst RS, et al. Association between tissue TMB (tTMB) and clinical outcomes with pembrolizumab monotherapy (pembro) in PD-L1-positive advanced NSCLC in the KEYNOTE-010 and -042 trials. Ann. Oncol. 2019;30:v851–v934. doi: 10.1093/annonc/mdy545. - DOI
-
- Peters S, et al. Abstract CT074: Tumor mutational burden (TMB) as a biomarker of survival in metastatic non-small cell lung cancer (mNSCLC): blood and tissue TMB analysis from MYSTIC, a phase III study of first-line durvalumab ± tremelimumab vs chemotherapy. Cancer Res. 2019;79:CT074–CT074.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

