α-Lipoic Acid Reduces Ceramide Synthesis and Neuroinflammation in the Hypothalamus of Insulin-Resistant Rats, While in the Cerebral Cortex Diminishes the β-Amyloid Accumulation
- PMID: 35422650
- PMCID: PMC9005076
- DOI: 10.2147/JIR.S358799
α-Lipoic Acid Reduces Ceramide Synthesis and Neuroinflammation in the Hypothalamus of Insulin-Resistant Rats, While in the Cerebral Cortex Diminishes the β-Amyloid Accumulation
Abstract
Background: Oxidative stress underlies metabolic diseases and cognitive impairment; thus, the use of antioxidants may improve brain function in insulin-resistant conditions. We are the first to evaluate the effects of α-lipoic acid (ALA) on redox homeostasis, sphingolipid metabolism, neuroinflammation, apoptosis, and β-amyloid accumulation in the cerebral cortex and hypothalamus of insulin-resistant rats.
Methods: The experiment was conducted on male cmdb/outbred Wistar rats fed a high-fat diet (HFD) for 10 weeks with intragastric administration of ALA (30 mg/kg body weight) for 4 weeks. Pro-oxidant and pro-inflammatory enzymes, oxidative stress, sphingolipid metabolism, neuroinflammation, apoptosis, and β-amyloid level were assessed in the hypothalamus and cerebral cortex using colorimetric, fluorimetric, ELISA, and HPLC methods. Statistical analysis was performed using three-way ANOVA followed by the Tukey HSD test.
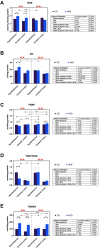
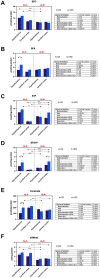
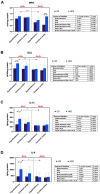
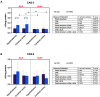
Results: ALA normalizes body weight, food intake, glycemia, insulinemia, and systemic insulin sensitivity in HFD-fed rats. ALA treatment reduces nicotinamide adenine dinucleotide phosphate (NADPH) and xanthine oxidase activity, increases ferric-reducing antioxidant power (FRAP) and thiol levels in the hypothalamus of insulin-resistant rats. In addition, it decreases myeloperoxidase, glucuronidase, and metalloproteinase-2 activity and pro-inflammatory cytokines (IL-1β, IL-6) levels, while in the cerebral cortex ALA reduces β-amyloid accumulation. In both brain structures, ALA diminishes ceramide synthesis and caspase-3 activity. ALA improves systemic oxidative status and reduces insulin-resistant rats' serum cytokines, chemokines, and growth factors.
Conclusion: ALA normalizes lipid and carbohydrate metabolism in insulin-resistant rats. At the brain level, ALA primarily affects hypothalamic metabolism. ALA improves redox homeostasis by decreasing the activity of pro-oxidant enzymes, enhancing total antioxidant potential, and reducing protein and lipid oxidative damage in the hypothalamus of HFD-fed rats. ALA also reduces hypothalamic inflammation and metalloproteinases activity, and cortical β-amyloid accumulation. In both brain structures, ALA diminishes ceramide synthesis and neuronal apoptosis. Although further study is needed, ALA may be a potential treatment for patients with cerebral complications of insulin resistance.
Keywords: brain; ceramide; inflammation; insulin resistance; oxidative stress; α-lipoic acid.
© 2022 Maciejczyk et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

Similar articles
-
α-Lipoic Acid Strengthens the Antioxidant Barrier and Reduces Oxidative, Nitrosative, and Glycative Damage, as well as Inhibits Inflammation and Apoptosis in the Hypothalamus but Not in the Cerebral Cortex of Insulin-Resistant Rats.Oxid Med Cell Longev. 2022 Mar 29;2022:7450514. doi: 10.1155/2022/7450514. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35391928 Free PMC article.
-
Redox Balance, Antioxidant Defense, and Oxidative Damage in the Hypothalamus and Cerebral Cortex of Rats with High Fat Diet-Induced Insulin Resistance.Oxid Med Cell Longev. 2018 Sep 6;2018:6940515. doi: 10.1155/2018/6940515. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 30271528 Free PMC article.
-
α -lipoic acid supplementation reduces oxidative stress and inflammation in red skeletal muscle of insulin-resistant rats.Biochem Biophys Res Commun. 2025 Jan;742:151107. doi: 10.1016/j.bbrc.2024.151107. Epub 2024 Dec 1. Biochem Biophys Res Commun. 2025. PMID: 39667068
-
Evaluation of the protective roles of alpha-lipoic acid supplementation on nanomaterial-induced toxicity: A meta-analysis of in vitro and in vivo studies.Front Nutr. 2022 Sep 6;9:991524. doi: 10.3389/fnut.2022.991524. eCollection 2022. Front Nutr. 2022. PMID: 36147302 Free PMC article.
-
The Multifaceted Role of Alpha-Lipoic Acid in Cancer Prevention, Occurrence, and Treatment.Antioxidants (Basel). 2024 Jul 25;13(8):897. doi: 10.3390/antiox13080897. Antioxidants (Basel). 2024. PMID: 39199143 Free PMC article. Review.
Cited by
-
Redox Biomarkers and Matrix Remodeling Molecules in Ovarian Cancer.Antioxidants (Basel). 2024 Feb 4;13(2):200. doi: 10.3390/antiox13020200. Antioxidants (Basel). 2024. PMID: 38397798 Free PMC article.
-
Endogenous and Exogenous Antioxidants in Skeletal Muscle Fatigue Development during Exercise.Antioxidants (Basel). 2023 Feb 16;12(2):501. doi: 10.3390/antiox12020501. Antioxidants (Basel). 2023. PMID: 36830059 Free PMC article. Review.
-
α-Lipoic acid: a potential regulator of copper metabolism in Alzheimer's disease.Front Mol Biosci. 2024 Sep 3;11:1451536. doi: 10.3389/fmolb.2024.1451536. eCollection 2024. Front Mol Biosci. 2024. PMID: 39290994 Free PMC article.
-
Biomarkers of traumatic brain injury: narrative review and future prospects in neurointensive care.Front Med (Lausanne). 2025 Jun 3;12:1539159. doi: 10.3389/fmed.2025.1539159. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40529152 Free PMC article. Review.
-
Antiglycoxidative properties of amantadine - a systematic review and comprehensive in vitro study.J Enzyme Inhib Med Chem. 2023 Dec;38(1):138-155. doi: 10.1080/14756366.2022.2137161. J Enzyme Inhib Med Chem. 2023. PMID: 36325591 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

