Altered Salivary Microbiota in Patients with Obstructive Sleep Apnea Comorbid Hypertension
- PMID: 35422668
- PMCID: PMC9005082
- DOI: 10.2147/NSS.S347630
Altered Salivary Microbiota in Patients with Obstructive Sleep Apnea Comorbid Hypertension
Abstract
Purpose: Microorganisms contribute to the pathogenesis of obstructive sleep apnea (OSA)-associated hypertension (HTN), while more studies focus on intestinal microbiome. However, the relationship between oral microbiota and OSA-associated HTN has yet to be elucidated. This study aimed to identify differences in salivary microbiota between patients with OSA comorbid HTN compared with OSA patients, and furthermore evaluate the relationship between oral microbiome changes and increased blood pressure in patients with OSA.
Patients and methods: This study collected salivary samples from 103 participants, including 27 healthy controls, 27 patients with OSA, 23 patients with HTN, and 26 patients with OSA comorbid HTN, to explore alterations of the oral microbiome using 16S rRNA gene V3-V4 high-throughput sequencing. And ultra-high-performance liquid chromatography was used for metabolomic analysis.
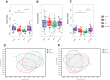
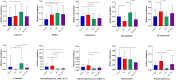
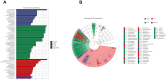
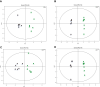
Results: Alpha- and beta-diversity analyses revealed a substantial difference in community structure and diversity in patients with OSA comorbid HTN compared with patients with OSA or HTN. The relative abundance of the genus Actinomyces was significantly decreased in patients with HTN compared with healthy controls, and those with OSA concomitant HTN compared with the patients in OSA, but was not significantly different between patients with OSA and healthy controls. Linear discriminant analysis effect size and variance analysis also indicated that the genera Haemophilus, Neisseria, and Lautropia were enriched in HTN. In addition, Oribacterium was an unique taxa in the OSA comorbid HTN group compared with the control group. Metabolomic analysis of saliva identified compounds associated with cardiovascular disease in patients with OSA comorbid HTN.2-hydroxyadenine, was significantly increased in the group of patients with OSA compared with controls, and L-carnitine was significantly decreased in patients with OSA comorbid HTN compared with OSA patients.
Conclusion: This study highlighted noninvasive biomarkers for patients with OSA comorbid HTN. As the first study to find alterations of the salivary microbiome in patients with OSA comorbid HTN, it may provide a theoretical foundation for clinical diagnosis and treatment of this condition.
Keywords: 16S rRNA; OSA; hypertension; metabolomics; oral microbiome.
© 2022 Chen et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest in this work.
Figures


References
-
- He QY, Feng J, Zhang XL, et al. Relationship of daytime blood pressure and severity of obstructive sleep apnea among Chinese: a multi-center investigation in China. Chin Med J. 2010;123(1):18–22. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

