Hypernatremia During Intravenous Treatment With Fosfomycin: A Retrospective Medical Record Review Study and an Analysis of Spontaneous Reports in the EudraVigilance Database
- PMID: 35422698
- PMCID: PMC9001889
- DOI: 10.3389/fphar.2022.844122
Hypernatremia During Intravenous Treatment With Fosfomycin: A Retrospective Medical Record Review Study and an Analysis of Spontaneous Reports in the EudraVigilance Database
Abstract
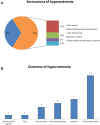
Background: Hypernatremia is a serious event that can occur during intravenous (IV) treatment with fosfomycin, and it can also be caused by a wrong drug preparation. Considering the clinical significance of hypernatremia, we decided to carry out two studies by using two different data sources with the aim to evaluate cases of IV fosfomycin-induced hypernatremia. Methods: A retrospective medical record review was performed from June 2017 to June 2019 using data from two hospitals in Southern Italy. The information collected was related to the patients, the antibiotic treatment regimen, type of adverse drug reaction (ADR), hypernatremia severity classification, and drug withdrawal due to ADRs. Moreover, a pharmacovigilance study was performed from the date of the European marketing authorization of fosfomycin to October 11, 2021, using data reported on the European website of suspected ADRs. Information related to the patient characteristics, treatment, hypernatremia, and type of reporter was retrieved. Results: From the retrospective medical record review, a total of 62 patients (48 men and 14 women) in treatment with fosfomycin were identified, of which 17 experienced ADRs. Specifically, 11 patients experienced hypernatremia. During the period from June 2017 to June 2018, a total of 63.7% of hypernatremia events were related to the wrong reconstitution of the drug. According to these results, a surveillance and training campaign about the correct drug reconstitution was managed. However, from June 2018 to June 2019, we still had four new hypernatremia cases. Drug withdrawal occurred in only one patient with hypernatremia. From the pharmacovigilance study, a total of 25 cases of IV fosfomycin-induced hypernatremia were retrieved. No substantial difference was found for patients' distribution by sex. Most cases were classified as serious (68%) and reported "Recovered/resolved" as the outcome (44%). In the majority of cases, fosfomycin was the only suspected drug reported (72%). Conclusion: Our results show that training campaigns on the correct drug preparation need to be strengthened to allow a reduction of hypernatremia cases. Moreover, when close monitoring and management is performed by the infectious disease (ID) specialist and hospital pharmacist, there also is a reduction in antibiotic withdrawal due to hypernatremia.
Keywords: ADR; EudraVigilance; hypernatremia; intravenous fosfomycin; retrospective study; safety.
Copyright © 2022 Scavone, Mascolo, Bernardi, Aiezza, Saturnino, Morra, Simonelli, Massa, Pomicino, Minei, Pisapia, Spatarella, Trama, Guglielmi, Capuano and Perrella.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials