Photobiont Diversity in Lichen Symbioses From Extreme Environments
- PMID: 35422771
- PMCID: PMC9002315
- DOI: 10.3389/fmicb.2022.809804
Photobiont Diversity in Lichen Symbioses From Extreme Environments
Abstract
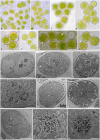
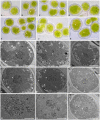
Fungal-algal relationships-both across evolutionary and ecological scales-are finely modulated by the presence of the symbionts in the environments and by the degree of selectivity and specificity that either symbiont develop reciprocally. In lichens, the green algal genus Trebouxia Puymaly is one of the most frequently recovered chlorobionts. Trebouxia species-level lineages have been recognized on the basis of their morphological and phylogenetic diversity, while their ecological preferences and distribution are still only partially unknown. We selected two cosmopolitan species complexes of lichen-forming fungi as reference models, i.e., Rhizoplaca melanophthalma and Tephromela atra, to investigate the diversity of their associated Trebouxia spp. in montane habitats across their distributional range worldwide. The greatest diversity of Trebouxia species-level lineages was recovered in the altitudinal range 1,000-2,500 m a.s.l. A total of 10 distinct Trebouxia species-level lineages were found to associate with either mycobiont, for which new photobionts are reported. One previously unrecognized Trebouxia species-level lineage was identified and is here provisionally named Trebouxia "A52." Analyses of cell morphology and ultrastructure were performed on axenically isolated strains to fully characterize the new Trebouxia "A52" and three other previously recognized lineages, i.e., Trebouxia "A02," T. vagua "A04," and T. vagua "A10," which were successfully isolated in culture during this study. The species-level diversity of Trebouxia associating with the two lichen-forming fungi in extreme habitats helps elucidate the evolutionary pathways that this lichen photobiont genus traversed to occupy varied climatic and vegetative regimes.
Keywords: Rhizoplaca; Tephromela; Trebouxia; chloroplast morphology; culture; phylogeny.
Copyright © 2022 De Carolis, Cometto, Moya, Barreno, Grube, Tretiach, Leavitt and Muggia.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Ahmadjian V. (1967). A guide to the algae occurring as lichen symbionts: isolation, culture, cultural physiology, and identification. Phycologia 6 127–160. 10.2216/i0031-8884-6-2-127.1 - DOI
-
- Beck A., Kasalicky T., Rambold G. (2002). Myco-photobiontal selection in a Mediterranean cryptogam community with Fulgensia fulgida. New Phytol. 153 317–326. 10.1046/j.0028-646x.2001.00315.x - DOI
-
- Bischoff H. W., Bold H. C. (1963). Phycological Studies IV. Some Soil Algae from Enchanted Rock and Related Algal Species. Univeristy of Texas Publication 6318. Austin, TX: University of Texas, 1–95.
-
- Blaha J., Baloch E., Grube M. (2006). High photobiont diversity associated with the euryoecious lichen-forming ascomycete Lecanora rupicola. Biol. J. Linn. Soc. 88 283–293.
LinkOut - more resources
Full Text Sources
Miscellaneous

