Explore the Mechanism of Astragalus mongholicus Bunge against Nonalcoholic Fatty Liver Disease Based on Network Pharmacology and Experimental Verification
- PMID: 35422858
- PMCID: PMC9005278
- DOI: 10.1155/2022/4745042
Explore the Mechanism of Astragalus mongholicus Bunge against Nonalcoholic Fatty Liver Disease Based on Network Pharmacology and Experimental Verification
Abstract
Objective: Astragalus mongholicus Bunge [Fabaceae] (AMB), a traditional Chinese medicine (TCM), has been widely used to treat liver diseases in the clinic. However, the efficacy and mechanism of AMB in the treatment of nonalcoholic fatty liver disease (NAFLD) remain unclear. The purpose of this study was to systematically investigate the active components and mechanisms of AMB against NAFLD based on network pharmacology, molecular docking, and experimental verification.
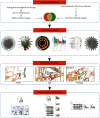
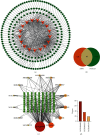
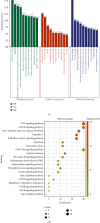
Methods: First, the bioactive components and relevant targets of AMB were screened from the Traditional Chinese Medicine Systematic Pharmacology (TCMSP) database, and NAFLD-related targets were obtained from the GeneCards database. Then, the AMB-NAFLD protein target interaction network was built by the STRING database. GO and KEGG pathway enrichment analyses were performed using the DAVID database. The component targets were visualized using Cytoscape software. Finally, molecular docking and experiments were used to verify the results of network pharmacological prediction.
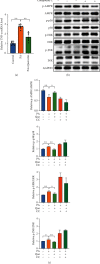
Results: Network pharmacology predicted that quercetin may be the main active component in AMB, and the TNF and MAPK signaling pathways may be the key targets of AMB against NAFLD. Molecular docking validation results demonstrated that quercetin, as the main active component of AMB, had the highest binding affinity with TNF. Furthermore, quercetin played a distinct role in alleviating NAFLD through in vitro experiments. Quercetin upregulated the phosphorylation levels of AMPK and inhibited the expression of p-MAPK and TNF-α. In addition, we further discovered that quercetin could increase ACC phosphorylation and CPT1α expression in PA-induced HepG2 cells.
Conclusions: Our results indicated that quercetin, as the main active component in AMB, exerts an anti-NAFLD effect by regulating the AMPK/MAPK/TNF-α and AMPK/ACC/CPT1α signaling pathways to inhibit inflammation and alleviate lipid accumulation.
Copyright © 2022 Lili Fu et al.
Conflict of interest statement
The authors declare no conflicts of interest for this work.
Figures






References
LinkOut - more resources
Full Text Sources

