Turning over on sticky balls: preparation and catalytic studies of surface-functionalized TiO2 nanoparticles
- PMID: 35423103
- PMCID: PMC8694772
- DOI: 10.1039/d0ra09319j
Turning over on sticky balls: preparation and catalytic studies of surface-functionalized TiO2 nanoparticles
Abstract
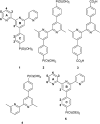
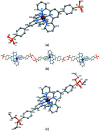
We have investigated the reactivity of rhodium(iii) complex-functionalized TiO2 nanoparticles and demonstrate a proof-of-principle study of their catalytic activity in an alcohol oxidation carried out under aqueous conditions water in air. TiO2 nanoparticles (NPs) have been treated with (4-([2,2':6',2''-terpyridin]-4'-yl)phenyl)phosphonic acid, 1, to give the functionalized NPs (1)@TiO2. Reaction between (1)@TiO2 NPs and either RhCl3·3H2O or [Rh2(μ-OAc)4(H2O)2] produced the rhodium(iii) complex-functionalized NPs Rh(1)2@TiO2. The functionalized NPs were characterized using thermogravimetric analysis (TGA), matrix-assisted laser desorption ionization (MALDI) mass spectrometry, 1H NMR and FT-IR spectroscopies; the single crystal structures of [Rh(1)2][NO3]3·1.25[H3O][NO3]·2.75H2O and of a phosphonate ester derivative were determined. 1H NMR spectroscopy was used to follow the reaction kinetics and to assess the recyclability of the NP-supported catalyst. The catalytic activity of the Rh(1)2@TiO2 NPs was compared to that of a homogeneous system containing [Rh(1)2]3+, confirming that no catalytic activity was lost upon surface-binding. Rh(1)2@TiO2 NPs were able to withstand reaction temperatures of up to 100 °C for 24 days without degradation.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




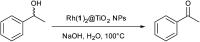



References
-
- Maleki A. Taheri-Ledari R. Ghalavand R. Firouzi-Haji R. J. Phys. Chem. Solids. 2020;136:109200. doi: 10.1016/j.jpcs.2019.109200. - DOI
-
- Hassan A. F. Elhadidy H. J. Phys. Chem. Solids. 2019;129:180–187. doi: 10.1016/j.jpcs.2019.01.018. - DOI
-
- Sajjadi S. Khataee A. Soltani R. D. C. Hasanzadeh A. J. Phys. Chem. Solids. 2019;127:140–150. doi: 10.1016/j.jpcs.2018.12.014. - DOI
-
- Zecchina A., Bordiga S. and Groppo E., Selective Nanocatalysts and Nanoscience, Wiley-VHC, Weinheim, 2011
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

