Tin-selenide as a futuristic material: properties and applications
- PMID: 35423185
- PMCID: PMC8694900
- DOI: 10.1039/d0ra09807h
Tin-selenide as a futuristic material: properties and applications
Abstract
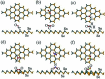
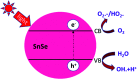
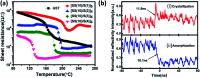
SnSe/SnSe2 is a promising versatile material with applications in various fields like solar cells, photodetectors, memory devices, lithium and sodium-ion batteries, gas sensing, photocatalysis, supercapacitors, topological insulators, resistive switching devices due to its optimal band gap. In this review, all possible applications of SnSe/SnSe2 have been summarized. Some of the basic properties, as well as synthesis techniques have also been outlined. This review will help the researcher to understand the properties and possible applications of tin selenide-based materials. Thus, this will help in advancing the field of tin selenide-based materials for next generation technology.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
Authors declare no conflict of interest exists.
Figures






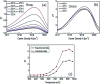
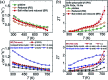
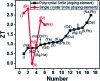


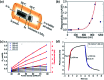

References
-
- Lokhande A. C. Qattan I. A. Lokhande C. D. Patole S. P. J. Mater. Chem. A. 2020;8:918–977. doi: 10.1039/C9TA10667G. - DOI
-
- Lokhande A. C. Babar P. T. Karade V. C. Gang M. G. Lokhande V. C. Lokhande C. D. Kim J. H. J. Mater. Chem. A. 2019;7:17118–17182. doi: 10.1039/C9TA00867E. - DOI
-
- Wang J. Chen R. Xiang L. Komarneni S. Ceram. Int. 2018;44:7357–7377. doi: 10.1016/j.ceramint.2018.02.013. - DOI
-
- Chung K. M. Wamwangi D. Woda M. Wuttig M. Bensch W. J. Appl. Phys. 2008;103:083523. doi: 10.1063/1.2894903. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

