Discovery of fragments inducing conformational effects in dynamic proteins using a second-harmonic generation biosensor
- PMID: 35423271
- PMCID: PMC8694943
- DOI: 10.1039/d0ra09844b
Discovery of fragments inducing conformational effects in dynamic proteins using a second-harmonic generation biosensor
Abstract
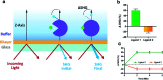
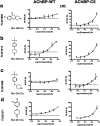
Biophysical screening of compound libraries for the identification of ligands that interact with a protein is efficient, but does typically not reveal if (or how) ligands may interfere with its functional properties. For this a biochemical/functional assay is required. But for proteins whose function is dependent on a conformational change, such assays are typically complex or have low throughput. Here we have explored a high-throughput second-harmonic generation (SHG) biosensor to detect fragments that induce conformational changes upon binding to a protein in real time and identify dynamic regions. Multiwell plate format SHG assays were developed for wild-type and six engineered single-cysteine mutants of acetyl choline binding protein (AChBP), a homologue to ligand gated ion channels (LGICs). They were conjugated with second harmonic-active labels via amine or maleimide coupling. To validate the assay, it was confirmed that the conformational changes induced in AChBP by nicotinic acetyl choline receptor (nAChR) agonists and antagonists were qualitatively different. A 1056 fragment library was subsequently screened against all variants and conformational modulators of AChBP were successfully identified, with hit rates from 9-22%, depending on the AChBP variant. A subset of four hits was selected for orthogonal validation and structural analysis. A time-resolved grating-coupled interferometry-based biosensor assay confirmed the interaction to be a reversible 1-step 1 : 1 interaction, and provided estimates of affinities and interaction kinetic rate constants (K D = 0.28-63 μM, k a = 0.1-6 μM-1 s-1, k d = 1 s-1). X-ray crystallography of two of the fragments confirmed their binding at a previously described conformationally dynamic site, corresponding to the regulatory site of LGICs. These results reveal that SHG has the sensitivity to identify fragments that induce conformational changes in a protein. A selection of fragment hits with a response profile different to known LGIC regulators was characterized and confirmed to bind to dynamic regions of the protein.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
M. B. & T. Y. were employees of Biodesy, Inc.
Figures





References
LinkOut - more resources
Full Text Sources

