Metal free C-3 chalcogenation (sulfenylation and selenylation) of 4 H-pyrido[1,2- a]pyrimidin-4-ones
- PMID: 35423521
- PMCID: PMC8695620
- DOI: 10.1039/d1ra00834j
Metal free C-3 chalcogenation (sulfenylation and selenylation) of 4 H-pyrido[1,2- a]pyrimidin-4-ones
Abstract
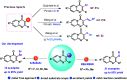
An expeditious metal free C-3 chalcogenation of 4H-pyrido[1,2-a]pyrimidin-4-one has been devised to synthesize diversely orchestrated 3-ArS/ArSe derivatives in high yields (up to 95%). This operationally simple reaction proceeds under mild reaction conditions, can be executed in gram scale, and also highlights broad functional group tolerance. Preliminary experimental investigation suggests a radical mechanistic pathway for these transformations.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
-
For selected examples:
- Mishra C. B. Kumari S. Tiwari M. Eur. J. Med. Chem. 2015;92:1. doi: 10.1016/j.ejmech.2014.12.031. - DOI - PubMed
- Ayati A. Emami S. Asadipour A. Shafiee A. Foroumadi A. Eur. J. Med. Chem. 2015;97:699. doi: 10.1016/j.ejmech.2015.04.015. - DOI - PubMed
- Sun P. Yang D. Wei W. Jiang L. Wang Y. Dai T. Wang H. Org. Chem. Front. 2017;4:1367. doi: 10.1039/C7QO00218A. - DOI
- Klečka M. Pohl R. Čejka J. Hocek M. Org. Biomol. Chem. 2013;11:5189. doi: 10.1039/C3OB40881G. - DOI - PubMed
-
-
-
For selected reviews and examples:
- Peach M. E., in The Chemistry of the Thiol Group, ed. S. Patai, John Wiley & Sons, London, 1979, pp. 721–756
- Damani L. A., Sulfur-Containing Drugs and Related Organic Compounds: Chemistry, Biochemistry, and Toxicology, Part B: Metabolism of Sulfur Functional Groups, Ellis Horwood Ltd, Chichester, UK, 1989, vol. 1
- Cremlyn R. J., in An Introduction to Organosulfur Chemistry, Wiley & Sons, New York, 1996
- McReynolds M. D. Doughtery J. M. Hanson P. R. Chem. Rev. 2004;104:2239. doi: 10.1021/cr020109k. - DOI - PubMed
- Zhao J. Jiang X. Chin. Chem. Lett. 2018;29:1079. doi: 10.1016/j.cclet.2018.05.026. - DOI
- Chauhan P. Mahajan S. Enders D. Chem. Rev. 2014;114:8807. doi: 10.1021/cr500235v. - DOI - PubMed
-
-
- Bellnier D. A. Young D. N. Detty M. R. Camacho S. Oseroff A. R. Photochem. Photobiol. 1999;70:630. doi: 10.1111/j.1751-1097.1999.tb08262.x. - DOI - PubMed
- Leonard K. A. Hall J. P. Nelen M. I. Davies S. R. Gollnick S. O. Camacho S. Oseroff A. R. Gibson S. L. Hilf R. Detty M. R. J. Med. Chem. 2000;43:4488. doi: 10.1021/jm000154p. - DOI - PubMed
-
- Li G. Gan Z. Kong K. Dou X. Yang D. Adv. Synth. Catal. 2019;361:1808. doi: 10.1002/adsc.201900157. - DOI
- Wei W. Wang L. Yue H. Jiang Y.-Y. Yang D. Org. Biomol. Chem. 2018;16:8379. doi: 10.1039/C8OB01349G. - DOI - PubMed
- Wang X. Yang M. Xie W. Fan X. Wu J. Chem. Commun. 2019;55:6010. doi: 10.1039/C9CC03004B. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

