Analysis of the effects of Cu-MOFs on fungal cell inactivation
- PMID: 35423710
- PMCID: PMC8693517
- DOI: 10.1039/d0ra08743b
Analysis of the effects of Cu-MOFs on fungal cell inactivation
Abstract
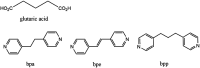
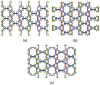
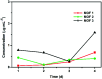
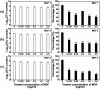
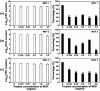
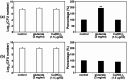
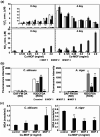
Three dimensional (3D) copper metal organic frameworks (Cu-MOFs) containing glutarates and bipyridyl ligands (bpa = 1,2-bis(4-pyridyl)ethane, bpe = 1,2-bis(4-pyridyl)ethylene, or bpp = 1,3-bis(4-pyridyl)propane) were synthesized by using previously reported hydrothermal reactions or a layering method. All three Cu-MOFs contained well-defined one dimensional (1D) channels with very similar pore shapes and different pore dimensions. The bulk purities of the Cu-MOFs were confirmed using powder X-ray diffraction (PXRD) and infrared spectroscopy (IR) spectra. When the three types of Cu-MOFs were applied to Candida albicans cells and Aspergillus niger spores, an average of about 50-80% inactivation was observed at the highest concentration of Cu-MOFs (2 mg mL-1). The efficiency of the fungal inactivation was not significantly different among the three different types (bpa, bpe, bpp). Treatment of the fungi using Cu-MOFs induced an apoptosis-like death and this was more severe in A. niger than C. albicans. This may be due to elevation of the intracellular level of reactive oxygen species (ROS) in A. niger. Generation of the reactive species in solution by Cu-MOFs was observed. However, there was a dramatic variation in the levels observed among the three types. Our results suggest that Cu-MOFs can produce antifungal effects and induce apoptosis-like death of the fungi, which was probably caused by the elevated level of intracellular reactive species.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


Similar articles
-
Bifunctional 3D Cu-MOFs containing glutarates and bipyridyl ligands: selective CO2 sorption and heterogeneous catalysis.Dalton Trans. 2012 Nov 7;41(41):12759-65. doi: 10.1039/c2dt31427d. Dalton Trans. 2012. PMID: 22968940
-
Antibacterial activities of Cu-MOFs containing glutarates and bipyridyl ligands.Dalton Trans. 2019 Jun 21;48(23):8084-8093. doi: 10.1039/c9dt00791a. Epub 2019 Apr 29. Dalton Trans. 2019. PMID: 31033965
-
Cobalt(II)-coordination polymers containing glutarates and bipyridyl ligands and their antifungal potential.Sci Rep. 2019 Oct 18;9(1):14983. doi: 10.1038/s41598-019-50258-1. Sci Rep. 2019. PMID: 31628353 Free PMC article.
-
Synthesis, structural diversity and fluorescent characterisation of a series of d10 metal-organic frameworks (MOFs): reaction conditions, secondary ligand and metal effects.Dalton Trans. 2011 Mar 21;40(11):2509-21. doi: 10.1039/c0dt01206h. Epub 2011 Feb 4. Dalton Trans. 2011. PMID: 21293812
-
Copper(II) 5-methoxyisophthalate coordination polymers incorporating dipyridyl co-ligands: syntheses, crystal structures, and magnetic properties.Dalton Trans. 2010 Mar 7;39(9):2301-8. doi: 10.1039/b920308g. Epub 2010 Jan 12. Dalton Trans. 2010. PMID: 20162204
Cited by
-
Metal Nanomaterials and Hydrolytic Enzyme-Based Formulations for Improved Antifungal Activity.Int J Mol Sci. 2023 Jul 12;24(14):11359. doi: 10.3390/ijms241411359. Int J Mol Sci. 2023. PMID: 37511117 Free PMC article. Review.
References
-
- Batten S. R., Turner D. R. and Neville S. M., Coordination Polymers: Design, Analysis and Application, RSC, London, 2009
- Design and Construction of Coordination Polymers, ed. M. C. Hong and L. Chen, Wiley, 2009
- Metal–Organic Frameworks: Design and Application, ed. L. R. MacGillivray, Wiley, 2010
- Metal–Organic Frameworks: Applications from Catalysis to Gas Storage, ed. D. Farrusseng, Wiley, 2011
- Functional Metal–Organic Frameworks: Gas Storage, Separation and Catalysis, ed. M. Schröder, Springer, 2010
- Ortiz O. L. and Ramírez L. D., Coordination Polymers and Metal Organic Frameworks: Properties, Types, and Applications, Nova Science Pub Inc., 2012
-
- Seayad A. M. Antonelli D. M. Recent advances in hydrogen storage in metal-containing inorganic nanostructures and related materials. Adv. Mater. 2004;16:765–777. doi: 10.1039/C2CC35418G. - DOI
- Eberle U. Felderhoff M. Schüth F. Chemical and physical solutions for hydrogen storage. Angew. Chem., Int. Ed. 2009;48:6608–6690. doi: 10.1039/C2CC35418G. - DOI
- Li J.-R. Ma Y. McCarthy M. C. Sculley J. Yu J. Jeong H.-K. Balbuena P. B. Zhou H.-C. Carbon dioxide capture-related gas adsorption and separation in metal-organic frameworks. Coord. Chem. Rev. 2011;255:1791–1823. doi: 10.1039/C2CC35418G. - DOI
- Bae Y.-S. Snurr R. Q. Development and evaluation of porous materials for carbon dioxide separation and capture. Angew. Chem., Int. Ed. 2011;50:11586–11596. doi: 10.1039/C2CC35418G. - DOI
- He Y. Zhou W. Krishnad R. Chen B. Microporous metal–organic frameworks for storage and separation of small hydrocarbons. Chem. Commun. 2012;48:11813–11831. doi: 10.1039/C2CC35418G. - DOI
- Sumida K. Rogow D. L. Mason J. A. McDonald T. M. Bloch E. D. Herm Z. R. Bae T.-H. Long J. R. Carbon dioxide capture in metal-organic frameworks. Chem. Rev. 2012;112:724–781. doi: 10.1039/C2CC35418G. - DOI
- Suh M. P. Park H. J. Prasad T. K. Lim D.-W. Hydrogen storage in metal-organic frameworks. Chem. Rev. 2012;112:782–835. doi: 10.1039/C2CC35418G. - DOI
- Foo M. L. Matsuda R. Kitagawa S. Functional hybrid porous coordination polymers. Chem. Mater. 2014;26:310–322. doi: 10.1039/C2CC35418G. - DOI
- Cadiau A. Adil K. Bhatt P. M. Belmabkhout Y. Eddaoudi M. A metal-organic framework–based splitter for separating propylene from propane. Science. 2016;353:137–140. doi: 10.1039/C2CC35418G. - DOI
- Cui X. Chen K. Xing H. Yang Q. Krishna R. Bao Z. Wu H. Zhou W. Dong X. Han Y. Li B. Ren Q. Zaworotko M. J. Chen B. Pore chemistry and size control in hybrid porous materials for acetylene capture from ethylene. Science. 2016;353:141–144. doi: 10.1039/C2CC35418G. - DOI
-
- Farrusseng D. Aguado S. Pinel C. Metal-organic frameworks: opportunities for catalysis. Angew. Chem., Int. Ed. 2009;48:7502–7513. doi: 10.1039/C1DT11274K. - DOI - PubMed
- Gu J.-M. Kim W.-S. Huh S. Size-dependent catalysis by DABCO-functionalized Zn-MOF with one-dimensional channels. Dalton Trans. 2011;40:10826–10829. doi: 10.1039/C1DT11274K. - DOI - PubMed
- Dhakshinamoorthy A. Opanasenko M. Čejka J. Garcia H. Metal organic frameworks as heterogeneous catalysts for the production of fine chemicals. Catal. Sci. Technol. 2013;3:2509–2540. doi: 10.1039/C1DT11274K. - DOI - PubMed
- Dias S. S. P. Kirillova M. V. André V. Kłak J. Kirillov A. M. New tricopper(II) cores self-assembled from aminoalcohol biobuffers and homophthalic acid: synthesis, structural and topological features, magnetic properties and mild catalytic oxidation of cyclic and linear C5-C8 alkanes. Inorg. Chem. Front. 2015;2:525–537. doi: 10.1039/C1DT11274K. - DOI - PubMed
- Gu J. Wen M. Cai Y. Shi Z. Arol A. S. Kirillova M. V. Kirillov A. M. Metal-Organic Architectures Assembled from Multifunctional Polycaboxylates: Hydrothermal Self-Assembly, Structures, and Catalytic Activity in Alkane Oxidation. Inorg. Chem. 2019;58:2403–2412. doi: 10.1039/C1DT11274K. - DOI - PubMed
-
- Yu Y. Ma J.-P. Zhao C.-W. Yang J. Zhang X.-M. Liu Q.-K. Dong Y.-B. Copper(I) Metal–Organic Framework: Visual Sensor for Detecting Small Polar Aliphatic Volatile Organic Compounds. Inorg. Chem. 2015;54:11590–11592. doi: 10.1039/C7TC03863A. - DOI - PubMed
- Jaros S. W. Sokolnicki J. Wołoszyn A. Haukka M. Kirillov A. M. Smoleński P. A novel 2D coordination network built from hexacopper(I)-iodide clusters and cagelike aminophosphine blocks for reversible ‘‘turn-on’’ sensing of aniline. J. Mater. Chem. C. 2018;6:1670. doi: 10.1039/C7TC03863A. - DOI - PubMed
-
- Horcajada P. Gref R. Baati T. Allan P. K. Maurin G. Couvreur P. Férey G. Morris R. E. Serre C. Metal-Organic Frameworks in Biomedicine. Chem. Rev. 2012;112:1232–1268. doi: 10.1021/cr200256v. - DOI - PubMed
- Cunha D. Yahia M. B. Hall S. Miller S. R. Chevreau H. Elkaïm E. Maurin G. Horcajada P. Serre C. Rationale of drug encapsulation and release from biocompatible porous metal–organic frameworks. Chem. Mater. 2013;25:2767–2776. doi: 10.1021/cr200256v. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

