Enhanced visible-light photodegradation of fluoroquinolone-based antibiotics and E. coli growth inhibition using Ag-TiO2 nanoparticles
- PMID: 35423911
- PMCID: PMC8697706
- DOI: 10.1039/d0ra10403e
Enhanced visible-light photodegradation of fluoroquinolone-based antibiotics and E. coli growth inhibition using Ag-TiO2 nanoparticles
Abstract
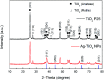
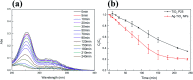
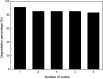
Antibiotics in wastewater represent a growing and worrying menace for environmental and human health fostering the spread of antimicrobial resistance. Titanium dioxide (TiO2) is a well-studied and well-performing photocatalyst for wastewater treatment. However, it presents drawbacks linked with the high energy needed for its activation and the fast electron-hole pair recombination. In this work, TiO2 nanoparticles were decorated with Ag nanoparticles by a facile photochemical reduction method to obtain an increased photocatalytic response under visible light. Although similar materials have been reported, we advanced this field by performing a study of the photocatalytic mechanism for Ag-TiO2 nanoparticles (Ag-TiO2 NPs) under visible light taking in consideration also the rutile phase of the TiO2 nanoparticles. Moreover, we examined the Ag-TiO2 NPs photocatalytic performance against two antibiotics from the same family. The obtained Ag-TiO2 NPs were fully characterised. The results showed that Ag NPs (average size: 23.9 ± 18.3 nm) were homogeneously dispersed on the TiO2 surface and the photo-response of the Ag-TiO2 NPs was greatly enhanced in the visible light region when compared to TiO2 P25. Hence, the obtained Ag-TiO2 NPs showed excellent photocatalytic degradation efficiency towards the two fluoroquinolone-based antibiotics ciprofloxacin (92%) and norfloxacin (94%) after 240 min of visible light irradiation, demonstrating a possible application of these particles in wastewater treatment. In addition, it was also proved that, after five Ag-TiO2 NPs re-utilisations in consecutive ciprofloxacin photodegradation reactions, only a photocatalytic efficiency drop of 8% was observed. Scavengers experiments demonstrated that the photocatalytic mechanism of ciprofloxacin degradation in the presence of Ag-TiO2 NPs is mainly driven by holes and ˙OH radicals, and that the rutile phase in the system plays a crucial role. Finally, Ag-TiO2 NPs showed also antibacterial activity towards Escherichia coli (E. coli) opening the avenue for a possible use of this material in hospital wastewater treatment.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Titanium Dioxide/Polyvinyl Alcohol/Cork Nanocomposite: A Floating Photocatalyst for the Degradation of Methylene Blue under Irradiation of a Visible Light Source.ACS Omega. 2021 May 25;6(22):14493-14503. doi: 10.1021/acsomega.1c01458. eCollection 2021 Jun 8. ACS Omega. 2021. PMID: 34124472 Free PMC article.
-
Highly efficient and stable Ag-AgBr/TiO2 composites for destruction of Escherichia coli under visible light irradiation.Water Res. 2013 Aug 1;47(12):4148-58. doi: 10.1016/j.watres.2012.11.057. Epub 2013 Mar 21. Water Res. 2013. PMID: 23562562
-
A novel P/Ag/Ag2O/Ag3PO4/TiO2 composite film for water purification and antibacterial application under solar light irradiation.Sci Total Environ. 2017 Jan 15;577:236-244. doi: 10.1016/j.scitotenv.2016.10.170. Epub 2016 Oct 31. Sci Total Environ. 2017. PMID: 27810300
-
Visible-Light Active Titanium Dioxide Nanomaterials with Bactericidal Properties.Nanomaterials (Basel). 2020 Jan 9;10(1):124. doi: 10.3390/nano10010124. Nanomaterials (Basel). 2020. PMID: 31936581 Free PMC article. Review.
-
Recent progress on Ag/TiO2 photocatalysts: photocatalytic and bactericidal behaviors.Environ Sci Pollut Res Int. 2021 Sep;28(33):44638-44666. doi: 10.1007/s11356-021-14996-y. Epub 2021 Jul 2. Environ Sci Pollut Res Int. 2021. PMID: 34212334 Free PMC article. Review.
Cited by
-
Efficient removal of methyl orange and ciprofloxacin by reusable Eu-TiO2/PVDF membranes with adsorption and photocatalysis methods.RSC Adv. 2024 Jun 10;14(26):18432-18443. doi: 10.1039/d4ra02962c. eCollection 2024 Jun 6. RSC Adv. 2024. PMID: 38860257 Free PMC article.
-
Enhanced antibacterial activity of acid treated MgO nanoparticles on Escherichia coli.RSC Adv. 2021 Nov 29;11(60):38202-38207. doi: 10.1039/d1ra06221b. eCollection 2021 Nov 23. RSC Adv. 2021. PMID: 35498104 Free PMC article.
-
Synthesis, characterization and photocatalytic properties of nanostructured lanthanide doped β-NaYF4/TiO2 composite films.Sci Rep. 2022 Aug 12;12(1):13748. doi: 10.1038/s41598-022-17256-2. Sci Rep. 2022. PMID: 35961994 Free PMC article.
-
Lignin-Derived Sustainable Nano-Platforms: A Multifunctional Solution for an Efficient Dye Removal.ChemSusChem. 2024 Dec 20;17(24):e202400841. doi: 10.1002/cssc.202400841. Epub 2024 Aug 27. ChemSusChem. 2024. PMID: 38899482 Free PMC article.
-
Multifunctional polymer-based nanocomposites for synergistic adsorption and photocatalytic degradation of mixed pollutants in water.Nanoscale Adv. 2023 Dec 4;6(6):1653-1660. doi: 10.1039/d3na00931a. eCollection 2024 Mar 12. Nanoscale Adv. 2023. PMID: 38482028 Free PMC article.
References
-
- Schwarzenbach P. Egli T. Hofstetter T. B. Von Gunten U. Wehrli B. Global water pollution and human health. Annu. Rev. Environ. Resour. 2010;35:109–136. doi: 10.1146/annurev-environ-100809-125342. - DOI
-
- Bhuiyan A. B. Mokhtar M. Toriman M. E. Gasim M. B. Ta G. C. Elfithri R. Razman M. R. The environmental risk and water pollution: A review from the river basins around the world. Am.-Eurasian J. Sustain. Agric. 2013;7:126–136.
-
- Durán-Álvarez J. C. Avella E. Ramírez-Zamora R. M. Zanella R. Photocatalytic degradation of ciprofloxacin using mono-(Au, Ag and Cu) and bi- (Au-Ag and Au-Cu) metallic nanoparticles supported on TiO2 under UV-C and simulated sunlight. Catal. Today. 2016;266:175–187. doi: 10.1016/j.cattod.2015.07.033. - DOI
-
- Ali T. Ahmed A. Alam U. Uddin I. Tripathi P. Muneer M. Enhanced photocatalytic and antibacterial activities of Ag-doped TiO2 nanoparticles under visible light. Mater. Chem. Phys. 2018;212:325–335. doi: 10.1016/j.matchemphys.2018.03.052. - DOI
LinkOut - more resources
Full Text Sources

