High efficiency solar desalination and dye retention of plasmonic/reduced graphene oxide based copper oxide nanocomposites
- PMID: 35424040
- PMCID: PMC8698584
- DOI: 10.1039/d1ra01663f
High efficiency solar desalination and dye retention of plasmonic/reduced graphene oxide based copper oxide nanocomposites
Abstract
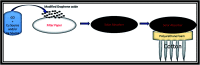
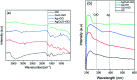
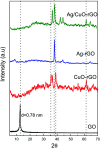
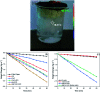
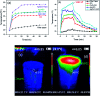
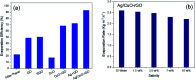
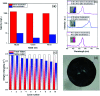
Water desalination via solar-driven interfacial evaporation is one of the most essential technologies to limit the problem of global freshwater scarcity. Searching for a highly efficient, stable, eco-friendly, and cost-effective solar-absorber material that can collect the full solar spectrum is critically important for solar steam generation. This study reports the development of a new solar thermal evaporation system based on plasmonic copper oxide/reduced graphene oxide (rGO). The silver nanoparticles in the composite exhibit a very strong solar absorption. Also, rGO and CuO nanoparticles offer excellent thermal absorptivity. Polyurethane was used as the support and as a thermal insulator. Moreover, filter paper was used for fast water delivery to the surface of the solar absorber. Ag/CuO-rGO nanocomposite is manifested to be one of the most efficient solar-absorbers reported to date for solar desalination which exhibits an average 2.6 kg m-2 h-1 evaporation rate with solar thermal efficiency up to 92.5% under 1 sun irradiation. Furthermore, the composite has excellent stability and durability as it displays stable evaporation rates for more than 10 repeated cycles in use, with no significant decrease in the activity. Besides, the successful removal of various organic dyes from contaminated water is also revealed, resulting in the production of clean condensed freshwater. Finally, this work commences a new avenue of synthesizing cost-effective thermal absorbers based on metal oxides.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
-
- Wu D. Zhao C. Xu Y. Zhang X. Yang L. Zhang Y. Gao Z. Song Y.-Y. ACS Appl. Nano Mater. 2020;3:10895–10904. doi: 10.1021/acsanm.0c02123. - DOI
-
- Chen L. Wang H. Kuravi S. Kota K. Park Y. H. Xu P. Desalination. 2020;483:114412. doi: 10.1016/j.desal.2020.114412. - DOI
-
- Gan Q. Zhang T. Chen R. Wang X. Ye M. ACS Sustainable Chem. Eng. 2019;7:3925–3932. doi: 10.1021/acssuschemeng.8b05036. - DOI
-
- Song L. Mu P. Geng L. Wang Q. Li J. ACS Sustainable Chem. Eng. 2020;8:11845–11852. doi: 10.1021/acssuschemeng.0c04407. - DOI
LinkOut - more resources
Full Text Sources

