Inhibitory properties of saponin from Eleocharis dulcis peel against α-glucosidase
- PMID: 35424054
- PMCID: PMC8698979
- DOI: 10.1039/d1ra02198b
Inhibitory properties of saponin from Eleocharis dulcis peel against α-glucosidase
Abstract
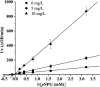
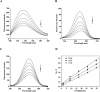
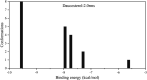
The inhibitory properties towards α-glucosidase in vitro and elevation of postprandial glycemia in mice by the saponin constituent from Eleocharis dulcis peel were evaluated for the first time. Three saponins were isolated by silica gel and HPLC, identified as stigmasterol glucoside, campesterol glucoside and daucosterol by NMR spectroscopy. Daucosterol presented the highest content and showed the strongest α-glucosidase inhibitory activity with competitive inhibition. Static fluorescence quenching of α-glucosidase was caused by the formation of the daucosterol-α-glucosidase complex, which was mainly derived from hydrogen bonds and van der Waals forces. Daucosterol formed 7 hydrogen bonds with 4 residues of the active site and produced hydrophobic interactions with 3 residues located at the exterior part of the binding pocket. The maltose-loading test results showed that daucosterol inhibited elevation of postprandial glycemia in ddY mice. This suggests that daucosterol from Eleocharis dulcis peel can potentially be used as a food supplement for anti-hyperglycemia.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors state that there is no conflict of interest.
Figures




References
-
- Stefano E. D. Oliviero T. Udenigwe C. C. Functional significance and structure-activity relationship of food-derived α-glucosidase inhibitors. Curr. Opin. Food Sci. 2018;20:7–12. doi: 10.1016/j.cofs.2018.02.008. - DOI
-
- Wang Q. Rehman M. Peng D. Liu L. Antioxidant capacity and α-glucosidase inhibitory activity of leaf extracts from ten ramie cultivars. Ind. Crops Prod. 2018;122:430–437. doi: 10.1016/j.indcrop.2018.06.020. - DOI
LinkOut - more resources
Full Text Sources

