Peptide modified manganese-doped iron oxide nanoparticles as a sensitive fluorescence nanosensor for non-invasive detection of trypsin activity in vitro and in vivo
- PMID: 35424166
- PMCID: PMC8693661
- DOI: 10.1039/d0ra08171j
Peptide modified manganese-doped iron oxide nanoparticles as a sensitive fluorescence nanosensor for non-invasive detection of trypsin activity in vitro and in vivo
Abstract
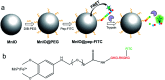
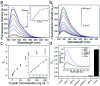
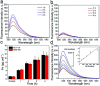
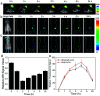
Herein, a fluorescence turn-on nanosensor (MnIO@pep-FITC) has been proposed for detecting trypsin activity in vitro and in vivo through covalently immobilizing an FITC modified peptide substrate of trypsin (pep-FITC) on manganese-doped iron oxide nanoparticle (MnIO NP) surfaces via a polyethylene glycol (PEG) crosslinker. The conjugation of pep-FITC with MnIO NPs results in the quenching of FITC fluorescence. After trypsin cleavage, the FITC moiety is released from the MnIO NP surface, leading to a remarkable recovery of FITC fluorescence signal. Under the optimum experimental conditions, the recovery ratio of FITC fluorescence intensity is linearly dependent on the trypsin concentration in the range of 2 to 100 ng mL-1 in buffer and intracellular trypsin in the lysate of 5 × 102 to 1 × 104 HCT116 cells per mL, respectively. The detection limit of trypsin is 0.6 ng mL-1 in buffer or 359 cells per mL HCT116 cell lysate. The MnIO@pep-FITC is successfully employed to noninvasively monitor trypsin activity in the ultrasmall (ca. 4.9 mm3 in volume) BALB/c nude mouse-bearing HCT116 tumor by in vivo fluorescence imaging with external magnetic field assistance, demonstrating that it has excellent practicability.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
LinkOut - more resources
Full Text Sources
Miscellaneous

