Concentration-dependent supramolecular self-assembly of A1/A2-asymmetric-difunctionalized pillar[5]arene
- PMID: 35424224
- PMCID: PMC8693802
- DOI: 10.1039/d1ra00078k
Concentration-dependent supramolecular self-assembly of A1/A2-asymmetric-difunctionalized pillar[5]arene
Abstract
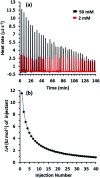
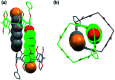
A series of A1/A2-bromoalkoxy-and-hydroxy-difunctionalized pillar[5]arenes were synthesized by the removal of the pillar[5]arene-bearing benzyl group using catalytic hydrogenation. The difunctionalized pillar[5]arene bearing 8-bromooctoxy and benzyloxy substituents at the A1/A2 positions formed pseudo[1]rotaxane at low concentration and double-threaded supramolecular dimer at high concentration. The supramolecular self-assembly behavior has been probed with multiple methods including varying (variable) concentration 1H NMR spectroscopy, diffusion-ordered spectroscopy (DOSY), dynamic light scattering (DLS) measurements, isothermal titration calorimetry (ITC), and single-crystal X-ray analysis.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
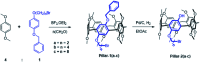



References
-
- Pairault N. Barat R. Tranoy-Opalinski I. Renoux B. Thomas M. Papot S. Rotaxane-Based Architectures for Biological Applications. C. R. Chim. 2016;19:103–112. doi: 10.1016/j.crci.2015.05.012. - DOI
-
- Brunsveld L. Folmer B. J. B. Meijer E. W. Sijbesma R. P. Supramolecular Polymers. Chem. Rev. 2001;101:4071–4098. doi: 10.1021/cr990125q. - DOI - PubMed
- Yang L. Tan X. Wang Z. Zhang X. Supramolecular Polymers: Historical Development, Preparation, Characterization, and Functions. Chem. Rev. 2015;115:7196–7239. doi: 10.1021/cr500633b. - DOI - PubMed
- Ogoshi T. Yamagishi T. Nakamoto Y. Pillar-Shaped Macrocyclic Hosts Pillar[n]arenes: New Key Players for Supramolecular Chemistry. Chem. Rev. 2016;116:7937–8002. doi: 10.1021/acs.chemrev.5b00765. - DOI - PubMed
- Al-Azemi T. F. Vinodh M. Pillar[5]arene-Based Self-Assembled Linear Supramolecular Polymer Driven by Guest Halogen–Halogen Interactions in Solid and Solution States. Polym. Chem. 2020;11:3305–3312. doi: 10.1039/D0PY00327A. - DOI
LinkOut - more resources
Full Text Sources

