Recent developments and perspectives in the copper-catalyzed multicomponent synthesis of heterocycles
- PMID: 35424324
- PMCID: PMC8694354
- DOI: 10.1039/d0ra10472h
Recent developments and perspectives in the copper-catalyzed multicomponent synthesis of heterocycles
Abstract
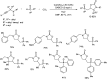
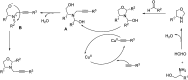
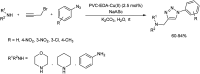
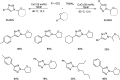
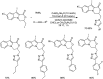
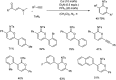
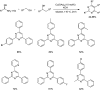
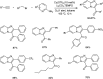
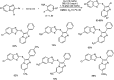
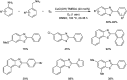
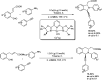
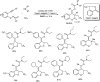
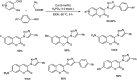
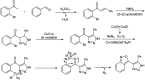
Heterocyclic compounds have become an inevitable part of organic chemistry due to their ubiquitous presence in bioactive compounds. Copper-catalyzed multicomponent synthesis of heterocycles has developed as the most convenient and facile synthetic route towards complex heterocyclic motifs. In this review, we discuss the advancements in the field of copper-catalyzed multicomponent reactions for the preparation of heterocycles since 2018.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures







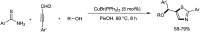




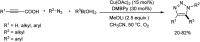











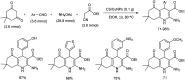

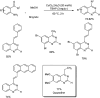
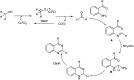

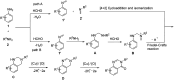









References
-
- Hu C. Lu L. Wan J. P. Curr. Med. Chem. 2017;24:2241. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

