Cinnamaldehyde-cucurbituril complex: investigation of loading efficiency and its role in enhancing cinnamaldehyde in vitro anti-tumor activity
- PMID: 35424684
- PMCID: PMC8982175
- DOI: 10.1039/d2ra00044j
Cinnamaldehyde-cucurbituril complex: investigation of loading efficiency and its role in enhancing cinnamaldehyde in vitro anti-tumor activity
Abstract
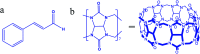
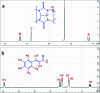
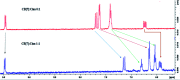
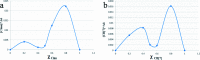
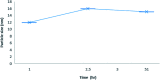
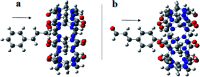
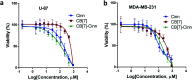
This study aimed to clarify the physico-chemical properties of cucurbit[7]uril (CB[7]) and cinnamaldehyde (Cinn) inclusion complexes (CB[7]-Cinn) and their resulting antitumor activity. CB[7]-Cinn inclusion complexes were prepared by a simple experimental approach and fully characterized for their stoichiometry, formation constant, particle size and morphology. Quantum chemical calculations were performed to elucidate the stable molecular structures of the inclusion complexes and their precursors and to investigate the probable stoichiometry and direction of interaction using three different DFT functionals at the 6-31G(d,p) basis set. The UV-vis spectrophotometric titrations as well as the Job plot, based on 1H NMR spectroscopy, suggested 1 : 1 and 1 : 2 stoichiometries of CB[7] : Cinn. The formation constants of the complexes were calculated using Benesi-Hildebrand equations and non-linear fittings. Moreover, the theoretical calculations confirmed the potential formation of 1 : 1 and 1 : 2 stoichiometries and clarify the orientation of binding from the Cinn phenyl moiety. The nanoparticles' TEM images showed a crystal-like spherical shape, smooth surface, with a small tendency to agglomerate. CB[7]-Cinn inclusion complexes were analyzed for their antitumor activity against MDA-MB-231 breast cancer and U-87 glioblastoma cell lines. The IC50 values were calculated after 72 hours of incubation with different concentrations of CB[7]-Cinn inclusion complexes and compared to free Cinn and free CB[7]. The IC50 values for free Cinn and CB[7]-Cinn inclusion complexes were 240.17 ± 32.46 μM and 260.47 ± 20.83 μM against U-87 cells and 85.93 ± 3.35 μM and 176.3 ± 7.79 μM against MDA-MB-231 cells, respectively, despite the enhanced aqueous solubility. No significant cytotoxicity was noticed for the free CB[7].
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


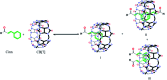




References
LinkOut - more resources
Full Text Sources
Miscellaneous

