Novel magnetic nickel ferrite nanoparticles modified with poly(aniline- co-o-toluidine) for the removal of hazardous 2,4-dichlorophenol pollutant from aqueous solutions
- PMID: 35424706
- PMCID: PMC8982154
- DOI: 10.1039/d2ra00034b
Novel magnetic nickel ferrite nanoparticles modified with poly(aniline- co-o-toluidine) for the removal of hazardous 2,4-dichlorophenol pollutant from aqueous solutions
Abstract
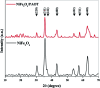
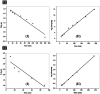
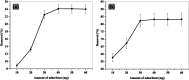
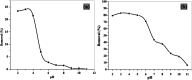
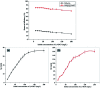
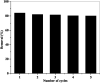
Chlorinated organic and phenolic compounds are still purely studied by many researchers because of their severe damage to the aquatic environment and their carcinogenic effect on many living organisms. Therefore, there is a great interest in removing these environmental pollutants from aqueous mediums by easy and inexpensive methods. Herein, novel nickel ferrite (NiFe2O4) nano composite modified with poly(aniline-co-o-toluidine) (PAOT) is prepared, characterized, and used for the removal of 2,4-dichlorophenol (2,4-DCP) as an organic chlorinated environmental pollutant. The morphological properties of the composite are characterized by Fourier transform infrared spectrometry (FTIR), X-ray diffraction (XRD), high-resolution transmission electron microscopy (HR-TEM), and Brunauer-Emmett-Teller (BET) methods. The prepared composite is tested for the removal of the hazardous dichlorophenol pollutant from aqueous solutions. Under optimized conditions and with effective control of parameters including, contact time, pH of the test solution, adsorbent dose, and temperature, over 83% of the pollutant is adsorbed and removed. The adsorption capacity is 162 mg g-1. Adsorption kinetics, adsorption isotherm and some physicochemical parameters of the reaction are evaluated. The Redlich-Peterson isothermal model is the appropriate model for describing the adsorption process. These results indicate that NiFe2O4/PAOT nanocomposites are promising adsorbents for the removal of persistent organic pollutants (e.g., DCP) from aqueous solutions. The results also reveal that modification of NiFe2O4 particles with poly(aniline-co-o-toluidine) (PAOT) significantly enhances the adsorption capacity of the adsorbent. This is probably due to the electrostatic attraction and non-covalent interactions (e.g. π-π) between the aromatic rings in both dichlorophenol and poly(aniline-co-o-toluidine) copolymer. Advantages offered by using NiFe2O4/PAOT nanocomposites are the high stability, reasonable efficiency, reusability for at least five adsorption-desorption cycles and the ability to remove the adsorbent from aqueous solutions for reuse using an external magnetic field.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that there are no conflicts of interest. All authors have approved the manuscript and agree with the submission to your esteemed journal.
Figures







References
-
- Li X. Zhou M. Pan Y. Xu L. Chem. Eng. J. 2017;307:1092–1104. doi: 10.1016/j.cej.2016.08.140. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

