Dispersing carbomers, mixing technology matters!
- PMID: 35424734
- PMCID: PMC8982170
- DOI: 10.1039/d2ra00176d
Dispersing carbomers, mixing technology matters!
Abstract
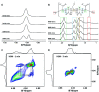
Mixing dry carbomer powder with water using magneto-hydrodynamic mixing yielded carbomer dispersions with higher viscosity and increased storage modulus as compared to conventional high shear mixing. 1H NMR spectroscopy demonstrated this to be induced by a different water distribution, accompanied by lower ionization and higher degradation of the polymer in case of high shear mixing. This investigation reveals 1H MAS NMR to provide suitable sensitivity and resolution to detect structural changes induced in organic polymers during their hydration.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Varges P. R. Costa C. M. Fonseca B. S. Naccache M. F. De Souza Mendes P. R. Fluids. 2019;4:3–22. doi: 10.3390/fluids4010003. - DOI
-
- Panzade P. Puranik P. K. Res. J. Pharm. Technol. 2010;3:672–675.
-
- Varghese S. A., Rangappa S. M., Siengchin S. and Parameswaranpillai J., in Hydrogels Based on Natural Polymers, ed. Y. Chen, Elsevier, 2020, pp. 17–47
-
- Silverson, Dispersion and Hydration of Carbopol®, https://www.silverson.com/us/resource-library/application-reports/disper..., accessed 1 June 2021
-
- Admix, Carbopol® Mixing/Carbomer Processing Equipment, https://www.admix.com/carbopol, accessed 28 May 2021
LinkOut - more resources
Full Text Sources

