2,5-Diisopropenylthiophene by Suzuki-Miyaura cross-coupling reaction and its exploitation in inverse vulcanization: a case study
- PMID: 35424896
- PMCID: PMC8985149
- DOI: 10.1039/d2ra00654e
2,5-Diisopropenylthiophene by Suzuki-Miyaura cross-coupling reaction and its exploitation in inverse vulcanization: a case study
Abstract
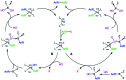
A novel thiophene derivative, namely 2,5-diisopropenylthiophene (DIT) was synthetized by Suzuki-Miyaura cross-coupling reaction (SMCCR). The influence of reaction parameters, such as temperature, solvent, stoichiometry of reagents, role of the base and reaction medium were thoroughly discussed in view of yield optimization and environmental impact minimization. Basic design of experiment (DoE) and multiple linear regression (MLR) modeling methods were used to interpret the obtained results. DIT was then employed as a comonomer in the copolymerization with waste elemental sulfur through a green process, inverse vulcanization (IV), to obtain sulfur-rich polymers named inverse vulcanized polymers (IVPs) possessing high refractive index (n ≈ 1.8). The DIT comonomer was purposely designed to (i) favor the IV process owing to the high reactivity of the isopropenyl functionalities and (ii) enhance the refractive index of the ensuing IVPs owing to the presence of the sulfur atom itself and to the high electronic polarizability of the π-conjugated thiophene ring. A series of random sulfur-r-diisopropenylthiophene (S-r-DIT) copolymers with sulfur content from 50 up to 90 wt% were synthesized by varying the S/DIT feed ratio. Spectroscopic, thermal and optical characterizations of the new IVPs were carried out to assess their main chemical-physical features.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
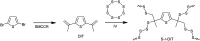





References
-
- Cross-coupling reactions: a practical guide, Topics in Current Chemistry Series 219, ed. N. Miyaura, Springer-Verlag, New York, USA, 2002
-
- Nobel Prize in Chemistry, 2010, available online, https://www.nobelprize.org/prizes/chemistry/2010/summary/, accessed on 10 December 2021
-
- Miyaura N. Suzuki A. Chem. Rev. 1995;95:2457–2483. doi: 10.1021/cr00039a007. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

