Rapid diagnosis of COVID-19 via nano-biosensor-implemented biomedical utilization: a systematic review
- PMID: 35424900
- PMCID: PMC8959446
- DOI: 10.1039/d2ra01293f
Rapid diagnosis of COVID-19 via nano-biosensor-implemented biomedical utilization: a systematic review
Abstract
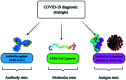
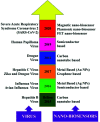
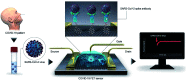
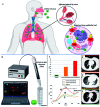
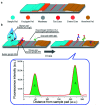
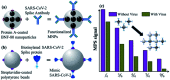
The novel human coronavirus pandemic is one of the most significant occurrences in human civilization. The rapid proliferation and mutation of Severe Acute Respiratory Syndrome-Coronavirus 2 (SARS-CoV-2) have created an exceedingly challenging situation throughout the world's healthcare systems ranging from underdeveloped countries to super-developed countries. The disease is generally recognized as coronavirus disease 2019 (COVID-19), and it is caused by a new human CoV, which has put mankind in jeopardy. COVID-19 is death-dealing and affects people of all ages, including the elderly and middle-aged people, children, infants, persons with co-morbidities, and immunocompromised patients. Moreover, multiple SARS-CoV-2 variants have evolved as a result of genetic alteration. Some variants cause severe symptoms in patients, while others cause an unusually high infection rate, and yet others cause extremely severe symptoms as well as a high infection rate. Contrasting with a previous epidemic, COVID-19 is more contagious since the spike protein of SARS-CoV-2 demonstrates profuse affection to angiotensin-converting enzyme II (ACE2) that is copiously expressed on the surface of human lung cells. Since the estimation and tracking of viral loads are essential for determining the infection stage and recovery duration, a quick, accurate, easy, cheap, and versatile diagnostic tool is critical for managing COVID-19, as well as for outbreak control. Currently, Reverse Transcription Polymerase Chain Reaction (RT-PCR) testing is the most often utilized approach for COVID-19 diagnosis, while Computed Tomography (CT) scans of the chest are used to assess the disease's stages. However, the RT-PCR method is non-portable, tedious, and laborious, and the latter is not capable of detecting the preliminary stage of infection. In these circumstances, nano-biosensors can play an important role to deliver point-of-care diagnosis for a variety of disorders including a wide variety of viral infections rapidly, economically, precisely, and accurately. New technologies are being developed to overcome the drawbacks of the current methods. Nano-biosensors comprise bioreceptors with electrochemical, optical, or FET-based transduction for the specific detection of biomarkers. Different types of organic-inorganic nanomaterials have been incorporated for designing, fabricating, and improving the performance and analytical ability of sensors by increasing sensitivity, adsorption, and biocompatibility. The particular focus of this review is to carry out a systematic study of the status and perspectives of synthetic routes for nano-biosensors, including their background, composition, fabrication processes, and prospective applications in the diagnosis of COVID-19.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures









Similar articles
-
Testing the efficacy and safety of BIO101, for the prevention of respiratory deterioration, in patients with COVID-19 pneumonia (COVA study): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Jan 11;22(1):42. doi: 10.1186/s13063-020-04998-5. Trials. 2021. PMID: 33430924 Free PMC article.
-
Current state of diagnostic, screening and surveillance testing methods for COVID-19 from an analytical chemistry point of view.Microchem J. 2021 Aug;167:106305. doi: 10.1016/j.microc.2021.106305. Epub 2021 Apr 19. Microchem J. 2021. PMID: 33897053 Free PMC article.
-
Research progress of biosensors for detection of SARS-CoV-2 variants based on ACE2.Talanta. 2023 Jan 1;251:123813. doi: 10.1016/j.talanta.2022.123813. Epub 2022 Aug 6. Talanta. 2023. PMID: 35952504 Free PMC article. Review.
-
COVID-19 diagnosis on the basis of nanobiosensors' prompt interactivity: A holistic review.Pathol Res Pract. 2024 Oct;262:155565. doi: 10.1016/j.prp.2024.155565. Epub 2024 Aug 29. Pathol Res Pract. 2024. PMID: 39226801 Review.
-
Comparative evaluation of RT-PCR and antigen-based rapid diagnostic tests (Ag-RDTs) for SARS-CoV-2 detection: performance, variant specificity, and clinical implications.Microbiol Spectr. 2024 Jun 4;12(6):e0007324. doi: 10.1128/spectrum.00073-24. Epub 2024 Apr 29. Microbiol Spectr. 2024. PMID: 38683014 Free PMC article.
Cited by
-
Noble Metal Nanoparticles for Point-of-Care Testing: Recent Advancements and Social Impacts.Bioengineering (Basel). 2022 Nov 8;9(11):666. doi: 10.3390/bioengineering9110666. Bioengineering (Basel). 2022. PMID: 36354576 Free PMC article. Review.
-
The Emergence of Carbon Nanomaterials as Effective Nano-Avenues to Fight against COVID-19.Materials (Basel). 2023 Jan 25;16(3):1068. doi: 10.3390/ma16031068. Materials (Basel). 2023. PMID: 36770075 Free PMC article. Review.
-
Bio-Inspired Nanomaterials for Micro/Nanodevices: A New Era in Biomedical Applications.Micromachines (Basel). 2023 Sep 18;14(9):1786. doi: 10.3390/mi14091786. Micromachines (Basel). 2023. PMID: 37763949 Free PMC article. Review.
-
Rapid assays of SARS-CoV-2 virus and noble biosensors by nanomaterials.Nano Converg. 2024 Jan 8;11(1):2. doi: 10.1186/s40580-023-00408-z. Nano Converg. 2024. PMID: 38190075 Free PMC article. Review.
-
Innovative Biosensing Approaches for Swift Identification of Candida Species, Intrusive Pathogenic Organisms.Life (Basel). 2023 Oct 22;13(10):2099. doi: 10.3390/life13102099. Life (Basel). 2023. PMID: 37895480 Free PMC article. Review.
References
-
- WHO, Transmission of SARS-CoV-2: implications for infection prevention precautions, 2020, available from https://www.who.int/news-room/commentaries/detail/transmission of SARS-C...
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

