Exploring a silicene monolayer as a promising sensor platform to detect and capture NO and CO gas
- PMID: 35424916
- PMCID: PMC8963267
- DOI: 10.1039/d2ra00442a
Exploring a silicene monolayer as a promising sensor platform to detect and capture NO and CO gas
Abstract
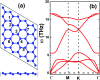
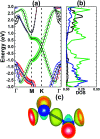
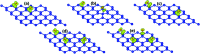
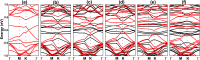
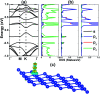
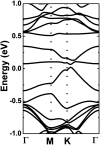
Searching for new two-dimensional (2D) materials for the early and efficient detection and capture of toxic gas has received special attention from researchers. In this work, we investigate the adsorption of NO and CO molecules onto a silicene monolayer using first-principles calculations. Different numbers of adsorbates, as well as adsorption configurations, have been considered. The results show that up to four NO molecules can be chemically adsorbed onto the pristine monolayer with adsorption energies varying between -0.32 and -1.22 eV per molecule. In these cases, the gas adsorption induces feature-rich electronic behaviors, including magnetic semiconducting and half-metallicity, where the magnetic properties are produced mainly by the adsorbates. Except for two CO molecules adsorbing onto two adjacent Si atoms with an adsorption energy of -0.26 eV per molecule, other adsorption configurations show weak physisorption of CO molecules onto the pristine silicene platform. However, the sensitivity can be enhanced considerably by doping with Al atoms, drastically reducing the adsorption energy to between -0.19 and -0.71 eV per molecule. The doping and adsorption process may lead to either band gap opening or metallization, depending on its configuration. This study reveals the promising applicability of pristine and Al doped silicene monolayers as sensors for more than one single NO and CO molecule.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Geim A. K. and Novoselov K. S., The rise of graphene, in Nanoscience and technology: a collection of reviews from nature journals, World Scientific, 2010, pp. 11–19
-
- Lee X. J. Hiew B. Y. Z. Lai K. C. Lee L. Y. Gan S. Thangalazhy-Gopakumar S. Rigby S. Review on graphene and its derivatives: synthesis methods and potential industrial implementation. J. Taiwan Inst. Chem. Eng. 2019;98:163–180. doi: 10.1016/j.jtice.2018.10.028. - DOI
-
- Chen D. Zhang H. Liu Y. Li J. Graphene and its derivatives for the development of solar cells, photoelectrochemical, and photocatalytic applications. Energy Environ. Sci. 2013;6(5):1362–1387. doi: 10.1039/C3EE23586F. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

