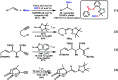
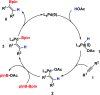
Palladium-catalyzed hydroboration reaction of unactivated alkynes with bis (pinacolato) diboron in water
- PMID: 35424934
- PMCID: PMC8961796
- DOI: 10.1039/d1ra09136k
Palladium-catalyzed hydroboration reaction of unactivated alkynes with bis (pinacolato) diboron in water
Abstract
A highly efficient and mild palladium-catalyzed hydroboration of unactivated internal alkynes in water is described. Both aryl- and alkyl-substituted alkynes proceeded smoothly within the reaction time to afford the desired vinylboronates in moderate to high yields. Bis (pinacolato) diboron was used to afford α- and β-hydroborated products in the presence of HOAc. These reactions showed high reactivities and tolerance, thus providing a promising method for the synthesis of alkenyl boron compounds.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
-
For reviews, see:
- Boronic Acids-Preparation and Applications in Organic Synthesis and Medicine, ed. D. G. Hall, Wiley-VCH Publishers, Weinheim, Germany, 2005
- Beletskaya I. Moberg C. Chem. Rev. 2006;106:2320–2354. doi: 10.1021/cr050530j. - DOI - PubMed
- Beletskaya I. Moberg C. Chem. Rev. 1999;99:3435–3461. doi: 10.1021/cr9902594. - DOI - PubMed
- Irvine G. J. Lesley M. J. G. Marder T. B. Norman N. C. Rice C. R. Robins E. G. Roper W. R. Whittell G. R. Wright L. J. Chem. Rev. 1998;98:2685–2722. doi: 10.1021/cr9500085. - DOI - PubMed
- Miyaura N. Suzuki A. Chem. Rev. 1995;95:2457–2483. doi: 10.1021/cr00039a007. - DOI
-
-
- Irving A. M. Vogels C. M. Nikolcheva L. G. Edwards J. P. He X.-F. Hamilton M. G. Baerlocher M. O. Baerlocher F. J. Decken A. Westcott S. A. New J. Chem. 2003;27:1419–1424. doi: 10.1039/B304500E. - DOI
- Ito H. Kawakami C. Sawamura M. J. Am. Chem. Soc. 2005;127:16034–16035. doi: 10.1021/ja056099x. - DOI - PubMed
- Krautwald S. Bezdek M. J. Chirik P. J. J. Am. Chem. Soc. 2017;139:3868–3875. doi: 10.1021/jacs.7b00445. - DOI - PMC - PubMed
-
-
For reviews, see:
- Suzuki A. in Organoboranes in syntheses, ACS symposium series 783, ed. P. V. Ramachandran and H. C. Brown, American Chemical Society, Washington, 2001, pp. 80–93, ch. 6
- Miyaura N. in Organoboranes in syntheses, ACS symposium series 783, ed. P. V. Ramachandran and H. C. Brown, American Chemical Society, Washington, 2001, pp. 94–107, ch. 7
- Palladium reagents and catalysts, ed. J. Tusiji, John Wiley & Sons Publishers, Chichester, England, 2014, pp. 218–227
-
-
- Boronic Acids, ed. D. G. Hall, Wiley-VCH Publishers, Weinheim, 2011
- Miyaura N. Suzuki A. Chem. Rev. 1995;95:2457. doi: 10.1021/cr00039a007. - DOI
- Kotha S. Lahiri K. Kashinath D. Tetrahedron. 2002;58:9633. doi: 10.1016/S0040-4020(02)01188-2. - DOI
- Molander G. A. Ellis N. Acc. Chem. Res. 2007;40:275. doi: 10.1021/ar050199q. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous