The development of imin-based tandem Michael-Mannich cyclocondensation through a single-electron transfer (SET)/energy transfer (EnT) pathway in the use of methylene blue (MB+) as a photo-redox catalyst
- PMID: 35425003
- PMCID: PMC8984404
- DOI: 10.1039/d2ra01190e
The development of imin-based tandem Michael-Mannich cyclocondensation through a single-electron transfer (SET)/energy transfer (EnT) pathway in the use of methylene blue (MB+) as a photo-redox catalyst
Abstract
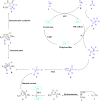
A four-component green tandem approach for the metal-free synthesis of polyfunctionalized dihydro-2-oxypyrroles was devised using the Michael-Mannich cyclocondensation of amines, dialkyl acetylenedicarboxylaes, and formaldehyde. Photo-excited state functions generated from methylene blue (MB+) were employed as single-electron transfer (SET) and energy transfer (EnT) catalysts at ambient temperature in an ethanol solvent, employing visible light as a renewable energy source in the air atmosphere. This study aims to increase the usage of a non-metal cationic dye that is both inexpensive and widely available. Methylene blue is photochemically produced with the least amount of a catalyst due to its high yields, energy-effectiveness, high atom economy, time-saving features of the reaction, and operational simplicity. As a result, a variety of ecological and long-term chemical features are achieved. Surprisingly, such cyclization can be done on a gram scale, implying that the process has industrial potential.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

