Metal-bio functionalized bismuthmagnetite [Fe3- x Bi x O4/SiO2@l-ArgEt3 +I-/Zn(ii)]: a novel bionanocomposite for the synthesis of 1,2,4,5-tetrahydro-2,4-dioxobenzo[ b][1,4]diazepine malononitriles and malonamides at room temperature and under sonication
- PMID: 35425005
- PMCID: PMC8972908
- DOI: 10.1039/d2ra00212d
Metal-bio functionalized bismuthmagnetite [Fe3- x Bi x O4/SiO2@l-ArgEt3 +I-/Zn(ii)]: a novel bionanocomposite for the synthesis of 1,2,4,5-tetrahydro-2,4-dioxobenzo[ b][1,4]diazepine malononitriles and malonamides at room temperature and under sonication
Abstract
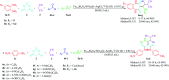
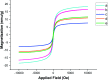
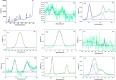
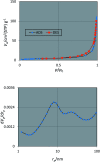
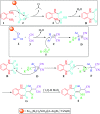
In this work, a new magnetized composite of bismuth (Fe3-x Bi x O4) was prepared and functionalized stepwise with silica, triethylargininium iodide ionic liquid, and Zn(ii) to prepare a multi-layered core-shell bio-nanostructure, [Fe3-x Bi x O4/SiO2@l-ArgEt3 +I-/Zn(ii)]. The modified bismuth magnetic amino acid-containing nanocomposite was characterized using several techniques including Fourier-transform infrared (FT-IR), X-ray fluorescence (XRF), vibrating sample magnetometer (VSM), field-emission scanning electron microscopy (FESEM), energy dispersive X-ray analysis (EDAX), thermogravimetric/differential scanning calorimetric (TGA/DSC) analysis, X-ray photoelectron spectroscopy (XPS), Brunauer-Emmett-Teller (BET), and inductively coupled plasma-optical emission spectrometry (ICP-OES). The magnetized bionanocomposite exhibited high catalytic activity for the synthesis of 1,2,4,5-tetrahydro-2,4-dioxobenzo[b][1,4]diazepine malononitriles via five-component reactions between 1,2-phenylenediamines, Meldrum's acid, malononitrile, aldehydes, and isocyanides at room temperature in ethanol. The efficacy of this protocol was also examined to obtain malonamide derivatives via pseudo six-component reactions of 1,4-phenylenediamine, Meldrum's acid, malononitrile, aldehydes, and isocyanides. When the above-mentioned MCRs were repeated under the same conditions with the application of sonication, a notable decrease in the reaction time was observed. The recovery and reusability of the metal-bio functionalized bismuthmagnetite were examined successfully in 3 runs. Furthermore, the characteristics of the recovered Fe3-x Bi x O4/SiO2@l-ArgEt3 +I-/Zn(ii) were investigated though FESEM and EDAX analysis.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






References
-
- Strecker A. Justus Liebigs Ann. Chem. 1850;75:27–45.
-
- Kouznetsov V. V. Galvi C. E. P. Tetrahedron. 2018;74:773–810.
- Weber L. Drug Discovery Today. 2002;7:143–147. - PubMed
-
- Ahmadi T. Mohammadi Ziarani G. Gholamzadeh P. Mollabagher H. Tetrahedron: Asymmetry. 2017;28:708–724.
- Nunes P. S. G. Vidal H. D. A. Corrêa A. G. Org. Biomol. Chem. 2020;18:7751–7773. - PubMed
LinkOut - more resources
Full Text Sources

