Coupling nucleic acid circuitry with the CRISPR-Cas12a system for universal and signal-on detection
- PMID: 35425009
- PMCID: PMC8977996
- DOI: 10.1039/d2ra01332k
Coupling nucleic acid circuitry with the CRISPR-Cas12a system for universal and signal-on detection
Abstract
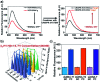
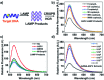
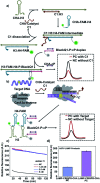
We report a universal and signal-on HCR based detection platform via innovatively coupling the CRISPR-Cas12a system with HCR. By using this CRISPR-HCR pathway, we can detect different targets by only changing the crRNA. The CRISPR-HCR platform coupling with an upstream amplifier can achieve a practical sensitivity as low as ∼aM of ASFV gene in serum.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources

