Antibacterial effect of copper nanoparticles produced in a Shewanella-supported non-external circuit bioelectrical system on bacterial plant pathogens
- PMID: 35425445
- PMCID: PMC8981026
- DOI: 10.1039/d1ra08187j
Antibacterial effect of copper nanoparticles produced in a Shewanella-supported non-external circuit bioelectrical system on bacterial plant pathogens
Abstract
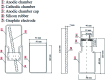
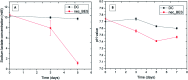
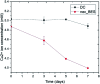
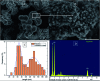
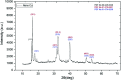
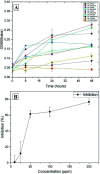
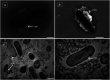
The use of copper nanoparticles for the inhibition of plant pathogens Ralstonia solanacearum, which causes wilt disease, and Xanthomonas axonopodis, which causes citrus canker, was investigated in this study. To avoid the inhibiting effect of Cu2+ ions on the bacterial cells, the copper nanoparticles were synthesized in the cathode chamber of a non-external circuit bioelectrochemical system (nec_BES) inoculated with Shewanella sp. HN-41 at the anode. The electrons produced by the oxidation of lactate by Shewanella sp. HN-41 were directly transferred to the anolyte in the cathode via a graphite electrode connecting the anode and cathode chambers. SEM images of the produced particles revealed that the copper nanoparticles were aggregated into spherical shapes with an average size of 2.9 μm from smaller particles with a size range from 30 nm to approximately 190 nm. X-ray diffraction demonstrated that the copper nanoparticles were mainly in the form of a single-phase crystal mixture of atacamite (Cu2Cl(OH)3) and paracatamite (Cu2Cl(OH)3). Finally, for the application of synthesized nanoparticles, an agar diffusion test was applied to assess the antibacterial activity of the formed copper nanoparticles in propylene glycol solvent against R. solanacearum and X. axonopodis. The results showed that the nanoparticles damaged the cells of R. solanacearum, with a half maximum inhibition (IC50) value of 42 ppm, but did not damage X. axonopodis cells.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The manuscript contains data and writing with no conflicts of interest.
Figures

References
-
- Fegan M., Haward A. C., Mark Fegan A. C. H., Liu D., Fegan M., Hayward A. and Mark Fegan A. C. H., in Manual of Security Sensitive Microbes and Toxins, CRC press, 2014, vol. 34, pp. 807–818
-
- Din M. I. Rehan R. Anal. Lett. 2017;50:50–62. doi: 10.1080/00032719.2016.1172081. - DOI
-
- Ghidan A. Y. and Antary T. M. A., in Applications of Nanobiotechnology, ed. M. Stoytcheva and R. Zlatev, IntechOpen, London, 2019, pp. 115–142
LinkOut - more resources
Full Text Sources

