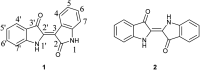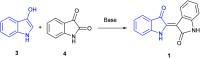A tunable synthesis of indigoids: targeting indirubin through temperature
- PMID: 35425542
- PMCID: PMC8981227
- DOI: 10.1039/d2ra00400c
A tunable synthesis of indigoids: targeting indirubin through temperature
Abstract
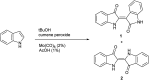
The spontaneous conversion of 3-indoxyl to indigo is a well-established process used to produce indigo dyes. It was recently shown that some indoles, when reacted with molybdenum hexacarbonyl and cumyl peroxide, proceed through an indoxyl intermediate to produce significant amounts of indirubin through a competing mechanism. Modulation of this system to lower temperatures allows for careful tuning, leading to selective production of indirubins in a general process. A systematic assay of indoles show that electron deficient indoles work well when substituted at the 5 and 7 positions. In contrast, 6-substituted electron rich indoles give the best results whereas halogeno indoles work well in all cases. This process shows broad functional group tolerance for generally reactive carbonyl-containing compounds such as aldehydes and carboxylic acids.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
References
-
- Jeong L. H. Sun-Young H. Pyeonghwa J. Yong-Chul K. Yeongyu M. Jungil C. Doo H. J. Pharmaceuticals. 2021;14:38. - PubMed
LinkOut - more resources
Full Text Sources